Figure 3. Cancer stem cell niche regulating plasticity, senescence and quiescence –
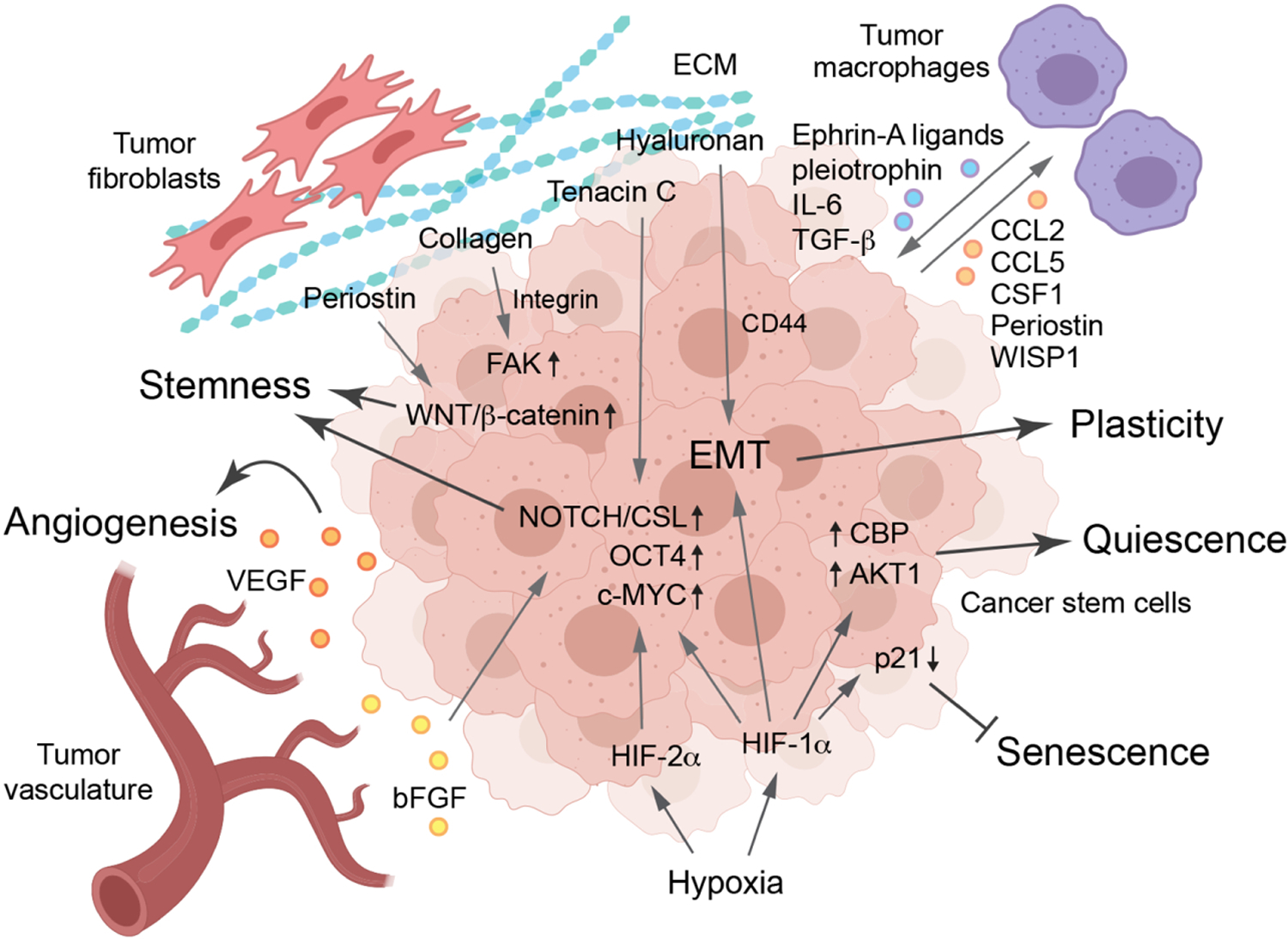
The niche of CSCs is defined by the support provided by their peripheral environment, namely, the vasculature, fibroblasts, and extracellular matrix (ECM), which is characterized by hypoxia. Hypoxia downregulates p21 to inhibit senescence, modulates Akt and CBP activity to induce cell quiescence, and activaes EMT and stem-cell pathways Notch/CSL, OCT4, and c-MYC to regulate plasticity and stemness. The CSCs and vasculature support each other by secreting various growth factors including VEGF and bFGF. ECM proteins, including periostin, collagen, hyaluronan, and tenacin-C, mainly derived from fibroblasts, activate stemness-activating pathways mediated through WNT/β-catenin, FAK, EMT, and NOTCH/CSL. Tumor-associated macrophages foster CSC phenotypes through ephrin-A ligands, pleiotrophin, IL-6, and TGF-β, and CSCs reciprocally induce macrophage recruitment and polarization through CCL2, CCL5, CSF1, periostin, and WISP1.
