Abstract
Aims
(1) To describe changes in arterial stiffness and heart rate variability (HRV) over a 5-year interval, (2) examine changes by sex and race–ethnicity, and (3) evaluate the risk factors associated with the longitudinal changes in arterial stiffness and HRV.
Methods
Participants with youth-onset type 2 diabetes enrolled in the observational follow-up phase of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial had arterial stiffness [(pulse wave velocity, augmentation index, brachial distensibility] and six indices of HRV measured 5 years apart. Multivariable linear regression models assessed risk factors associated with changes in the outcomes over time.
Results
At initial vascular assessment, the 304 participants were a mean age of 21 years, 34% male, and had a mean diabetes duration of 8 years. In more than half the cohort pulse wave velocity, augmentation index and HRV increased over 5 years (p<0.01). Brachial distensibility did not change. There were no differences in the 5-year change by race/ethnicity except for a single HRV measure, where non-Hispanic Blacks had greater worsening of parasympathetic function compared to non-Hispanic Whites, p = 0.008. Blood pressure was related to greater worsening in augmentation index and pulse wave velocity. Higher hemoglobin A1c over time was related to worsening pulse wave velocity and HRV.
Conclusions
Arterial stiffness and HRV worsened over 5 years. Blood pressure and glycemic control may be potential targets to influence adverse changes in arterial stiffness and HRV in young adults with youth-onset type 2 diabetes.
Keywords: Arterial stiffness, Heart rate variability, Type 2 diabetes, Risk factors
Introduction
Individuals with youth-onset type 2 diabetes appear to be at high risk to develop diabetes-related cardiovascular complications based on cross-sectional data [1–5]. Compared to individuals with youth-onset type 1 diabetes or their obese peers, adolescents and young adults with youth-onset type 2 diabetes have greater arterial stiffness and more abnormalities in heart rate variability [1–5]. Arterial stiffness is an early marker of cardiovascular disease and in adults predicts greater cardiovascular morbidity and mortality [6, 7]. Abnormalities in heart rate variability represent cardiac autonomic dysfunction and are associated with arrhythmia, myocardial infarction, and sudden death independent of other cardiovascular risk factors [8–12]. Furthermore, alterations in heart rate variability have been associated with increased arterial stiffness in individuals with diabetes [13, 14].
Studies in adults with and without diabetes have described worsening of arterial stiffness, and heart rate variability as well as their associated risk factors [6, 15, 16]. However, there were limited data in adolescence and young adults. Recently, Ryder et al. [17] found arterial stiffness was accelerated in youth with type 2 diabetes, mean age 17 years, compared to obese and lean controls over a 5-year period. However, heart rate variability was not assessed in that study and the cohort was largely limited to non-Hispanic White and non-Hispanic Black adolescents [17].
Here, we examined changes in arterial stiffness and heart rate variability over time in participants enrolled in the observational follow-up phase of the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial, a multi-ethnic study of adolescents with youth-onset type 2 diabetes [18, 19]. The objectives of this study were to: (1) describe changes in arterial stiffness and heart rate variability over a 5-year follow-up period in participants enrolled in the observational follow-up phase of the TODAY clinical trial; (2) examine sex and race–ethnicity differences in the change in arterial stiffness and heart rate variability over time; and (3) assess risk factors associated with changes in arterial stiffness and heart rate variability during the 5-year follow-up.
Materials and methods
TODAY participants and study design
Detailed descriptions of the TODAY clinical trial protocol (www.clinicaltrials.gov : NCT00081328) and the primary outcome results have been published [18–20]. In brief, the TODAY study (2004–2011) was a multicenter randomized clinical trial designed to assess metformin monotherapy, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention on time to treatment failure defined as loss of glycemic control (hemoglobin A1c ≥ 8% [64 mmol/mol] for 6 months or inability to wean from temporary insulin after acute metabolic decompensation) in adolescents with youth-onset type 2 diabetes. Participants in TODAY were followed for an average of 3.9 years [range 2–6 years]. At the end of the TODAY study, 572 of the original 699 TODAY participants enrolled in the TODAY observational follow-up study (TODAY2) designed to assess the long-term complications of diabetes.
The TODAY2 observational follow-up study was conducted between 2011 and 2020. During this time, participants were invited to 19 study visits (quarterly during 2011–2014 and annually during 2014–2020). Arterial stiffness and/or heart rate variability measurement were assessed at two of the 19 study visits, first at 7.1 ± 1.3 years in the TODAY study and then again after 5 years. The study was approved by institutional review boards at all 15 centers, and all participants and guardians provided written informed assent and/or consent as appropriate for age and local guidelines.
Risk factor assessment
Race–ethnicity was self-reported using U.S. Census-based questions. The anthropometrics and laboratory data were obtained at or within 1 month of the arterial stiffness and heart rate variability assessments. Medical history including medication usage was obtained, and a study-wide protocol to measure blood pressure, weight, and height was used [18]. Mean arterial pressure was calculated as [(2*diastolic blood pressure) + systolic blood pressure]/3.
Participants self-reported cigarette smoking was categorized as either ‘yes’ (used within the past month) or ‘no’ (never used/not used within the past month). Hemoglobin A1c was measured at the TODAY Central Biochemistry Laboratory (Seattle, WA) during every visit using a dedicated high-performance liquid chromatography method (TOSOH Biosciences Inc., South San Francisco, CA). Hemoglobin A1c and fasting labs were obtained according to standardized procedures and shipped to the Central Biochemistry Laboratory [18].
Outcome measures
Arterial stiffness outcomes included (1) carotid femoral pulse wave velocity (primary outcome), (2) carotid radial pulse wave velocity, (3) femoral foot pulse wave velocity, (4) augmentation index, and (5) brachial distensibility. Heart rate variability indices included three time domains (1) the SD of the R–R interval (termed NN interval, SDNN-primary and an overall measure of heart rate variability) from an electrocardiogram, (2) the root-mean-square differences of successive NN intervals (RMSSD), and (3) the percent of adjacent NN intervals with a difference greater than 50msec (PNN50). Heart rate variability also includes three frequency domains: (1) normalized low-frequency power, (2) high-frequency power, and (3) their ratio low frequency/high frequency. Detailed descriptions of each method have been published [3, 5].
All three pulse wave velocity, augmentation index and heart rate variability measurements were obtained using the SphygmoCor CPV system (AtCor Medical, Lisle, IL). Brachial distensibility was measured using the DynaPulse 2000 (PulseMetric, San Diego, CA). All measurements were conducted fasting and after the participant rested for at least 10 minutes. All prescriptions and over-the-counter medications were held on the day of testing until both tests were complete. TODAY staff were certified for performance of all procedures by a central Vascular Reading Center located in Cincinnati, Ohio.
Statistical analyses
Descriptive statistics are presented as mean ± SD, median [25th–75th percentile] or percent. Paired t-tests for continuous variables and McNemar tests for categorical variables were used to evaluate differences in participant characteristics between the initial and follow-up visit. The overall 5-year change (slope) from the initial to the follow-up assessment for each outcome was estimated from linear regression models adjusted for age, sex, and race–ethnicity. Similar models were used to evaluate differences in the slopes (change) by subgroup (sex, race–ethnicity, and randomized treatment group), via the addition of an interaction term of time (initial or follow-up). All the arterial stiffness outcomes as well as two of the heart rate variability outcomes (high-frequency power and low-frequency power) were approximately normally distributed. Other heart rate variability outcomes (SDNN, RMSSD, PNN50, and low-frequency/high-frequency ratio) were skewed and required a log transformation prior to testing to approximate normality.
Separately, multivariable linear regression models were used to relate risk factors with changes in arterial stiffness and heart rate variability outcomes. For each outcome, the change (follow-up minus initial value) was included as the dependent variable with an adjustment in the model for the initial value of the assessment along with other fixed covariate(s) as specified below. Covariates included a mix of continuous, binary, or categorical measurements from the 5-year follow-up assessment (time-weighted mean hemoglobin A1c, age, sex, race–ethnicity, body mass index, low-density lipoprotein cholesterol, and mean arterial pressure only in the arterial stiffness models to account for baseline distending pressure of the artery). Smoking was defined as having smoked at least one cigarette in the past month. Given that hemoglobin A1c was measured a median number of 7 times during the 5-year interval between each arterial stiffness/heart rate variability measurement, a time-weighted mean hemoglobin A1c (computed weighting each value by the time interval between assessments) was used to represent the glycemic exposure. Risk factors were selected given our prior cross-sectional associations with arterial stiffness and heart rate variability measurements in this cohort [3, 5]. Height was also included in the augmentation index model and heart rate (beats per minute) in the models of pulse wave velocity, and brachial distensibility. Heart rate was not included in the augmentation index model since heart rate was already adjusted to a standard 75 beats per minute. R-square and p-value of the multivariable models were evaluated to assess the robustness of the models. Statistical significance was defined as p<0.05. All analyses are considered exploratory; therefore no adjustments were made for multiple testing.
Results
Participants
The analyses reported here include data on 304 participants at all 15 clinical sites who had at least one arterial stiffness or heart rate variability measurement at an initial (after 7.1 ± 1.3 years in the study) and a 5-year follow-up study visit (after 12.1 ± 1.3 years of study). Participants excluded in these analyses had either missing initial or follow-up arterial stiffness or heart rate variability data (n=117). Participants with missing data did not differ by age, sex, race–ethnicity, body mass index, blood pressure, or hemoglobin A1c from the 304 included in this analysis. However, there was a lower proportion of Hispanic participants in those excluded versus included (25.6% vs 42.1% p=0.01). Eleven participants with monogenic diabetes mutations were excluded.
Arterial stiffness overtime
Metabolic and cardiovascular risk factors at the initial and follow-up arterial stiffness and heart rate variability assessment are presented in Table 1. Mean arterial stiffness, measured by carotid femoral pulse wave velocity, carotid radial pulse wave velocity, femoral foot pulse wave velocity and augmentation index, increased between 14.2 and 57.8% over 5 years, p<0.01 (Fig. 1 and Additional file 1: Data S1). This reflects a mean annualized change in carotid femoral pulse wave velocity of 0.15m/s per year, carotid radial pulse wave velocity of 0.24m/s per year, femoral foot pulse wave velocity of 0.15m/s per year, and augmentation index of 1.10% per year. Although the change in mean brachial distensibility was not significant, brachial distensibility worsened in nearly 50% of the cohort.
Table 1.
Characteristics of the TODAY participants at the initial arterial stiffness and heart rate variability assessment (2013–2014) and at the time of the 5-year follow-up assessment (2018–2019), N=304
| Characteristic | Initial assessment | 5-year follow-up assessment | P-value |
|---|---|---|---|
| Age (years) | 20.8 ± 2.5 | 25.9 ± 2.5 | n/a |
| Duration of diabetes (years) | 7.7 ± 1.5 | 12.8 ± 1.5 | n/a |
| Male (%) | 33.6% | -- | n/a |
| Race–ethnicity (%) | |||
| Non-Hispanic Black | 32.9% | -- | n/a |
| Hispanic | 42.1% | -- | |
| Non-Hispanic White | 18.4% | -- | |
| Other | 6.6% | -- | |
| Hemoglobin A1c (%) | 8.8 ± 2.8 | 9.6 ± 2.7 | < 0.0001 |
| Any diabetes medication use (%) | 93.1% | 75.7% | < 0.0001 |
| On metformin (%) | 85.5% | 51.6% | < 0.0001 |
| On insulin (%) | 66.8% | 53.6% | < 0.0001 |
| Body mass index (kg/m2) | 36.8 ± 8.4 | 36.1 ± 8.4 | 0.03 |
| Systolic blood pressure (mm Hg) | 118.5 ± 12.5 | 124.1 ± 15.1 | < 0.0001 |
| Diastolic blood pressure (mm Hg) | 73.3 ± 9.9 | 76.7 ± 11.0 | < 0.0001 |
| Anti-hypertensive medication use (%) a | 47.0% | 36.5% | 0.0004 |
| Total cholesterol (mg/dL) | 167.7 ± 36.9 | 182.3 ± 45.3 | < 0.0001 |
| Low-density lipoprotein cholesterol (mg/dL) | 98.8 ± 31.2 | 105.6 ± 36.3 | 0.0002 |
| High-density lipoprotein cholesterol (mg/dL) | 42.8 ± 11.5 | 46.4 ± 12.9 | < 0.0001 |
| Triglycerides (mg/dL) | 103 [71, 154] | 108 [77, 177] | 0.003 |
| Lipid-lowering medication use (%) | 15.8% | 13.8% | 0.42 |
| Smoking in past month, any (%) | 20.3% | 23.0% | 0.19 |
| Measured heart rate (beats/min) | 79.0 ± 11.1 | 79.7 ± 12.2 | 0.31 |
Mean ± SD, median [25th–75th percentile] or percent
P-value testing paired differences between the initial versus the 5-year follow-up assessment, using a paired t-test for continuous measures or the McNemar’s test for binary measures
n/a not applicable
Primarily ACE inhibitors and ARB
Figure 1.
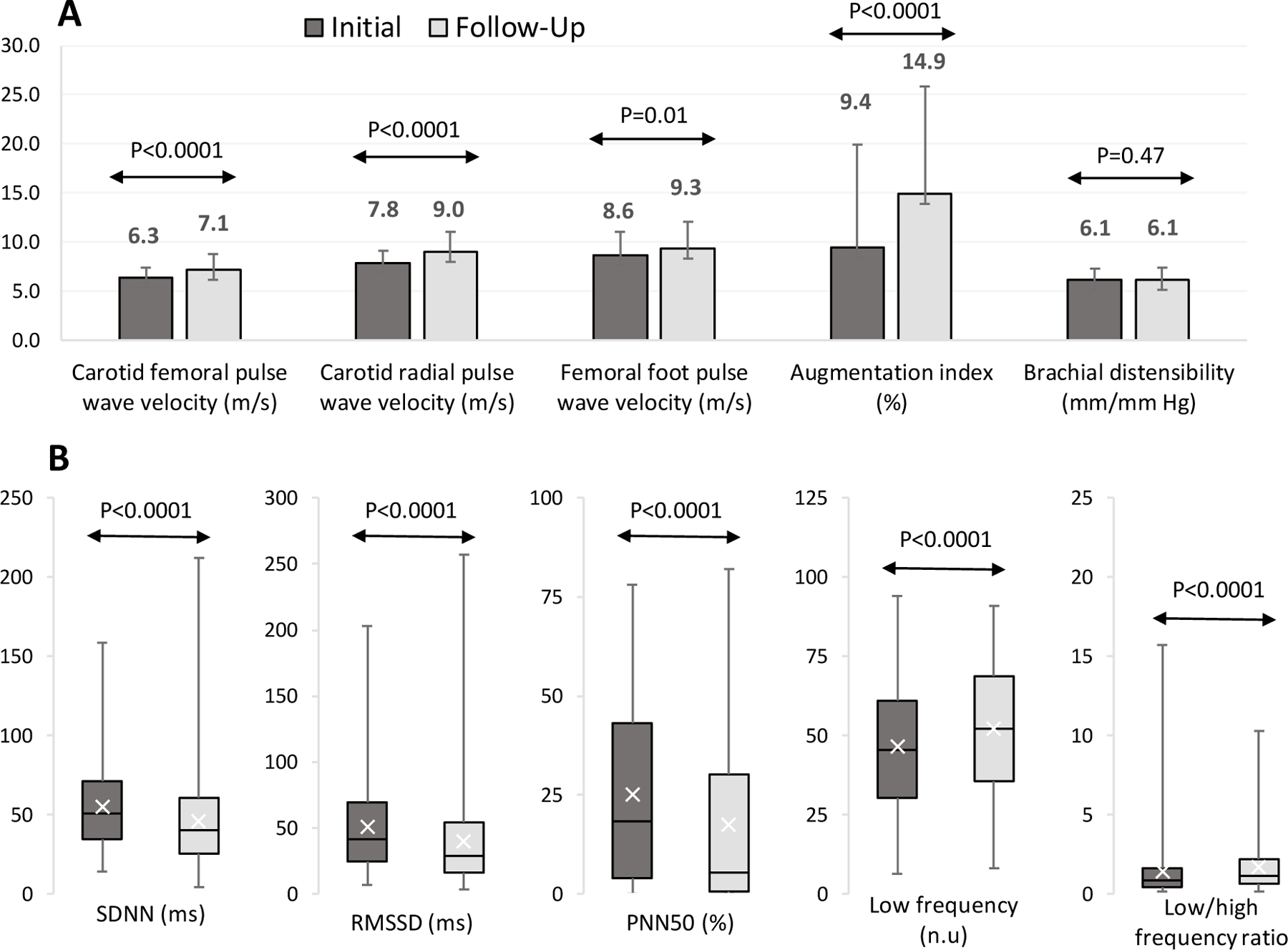
Arterial Stiffness (Panel A) and Heart Rate Variability (Panel B) Outcomes in the TODAY Participants (N=304) at the Initial (2013–2014) and 5-Year Follow-Up (2018–2019) Assessment*
Panel A: Mean ± SD at initial and 5-year follow-up assessment for the arterial stiffness measures. Panel B: median [25th −75th percentile] at initial and 5-year follow-up assessment for the heart rate variability measures. SDNN: SD of the NN intervals; RMSSD: root mean square differences of successive NN intervals; PNN50: percent of adjacent NN intervals with a difference greater than 50msec; n.u.normalized units. * P-value testing paired differences between the initial vs. the 5-year follow-up assessment, using at-test for continuous measures from adjusted models for age, sex, and race-ethnicity. Higher value is worse for all arterial stiffness measures except brachial distensibility, for low frequency, and for low frequency/high frequency ratio; Lower value is worse for all others.
Heart rate variability overtime
There was also worsening of overall heart rate variability (SDNN) and each of the heart rate variability indices, all p<0.0001 (Fig. 1 and Additional file 1: Data S1). The median change in heart rate variability was between 10.1 and 50.0% (p<0.0001). At the first assessment, 73.0% of the cohort had a SDNN of < 70ms, a value associated with a 4-fold higher risk of death in adults [21]. At the second heart rate variability assessment, an SDNN < 70ms was seen in 83.5% of participants.
Differences by race/ethnicity or prior diabetes treatment
The observed changes in arterial stiffness and heart rate variability did not differ by sex or race–ethnicity for any of the outcomes, except for RMSSD, where the change in non-Hispanic Blacks (− 15.3 ± 35.1 ms) was significantly greater than the change in non-Hispanic Whites (− 4.6 ± 39.9 ms, p=0.008). See Additional file 1: Data S2. Arterial stiffness and heart rate variability measurements did not differ over time by prior randomized treatment group (metformin alone, metformin with lifestyle, or metformin with rosiglitazone) or by loss of glycemic control (hemoglobin A1c ≥ 8% [64 mmol/mol]) during the study, data not shown.
Risk factors that influence change overtime
Associations of risk factors with changes in arterial stiffness during TODAY2 are shown in Table 2. HigherA time-weighted hemoglobin A1c and mean arterial pressure were positively associated with a change in carotid femoral pulse wave velocity over time; however, there was no significant interaction between hemoglobin A1c and mean arterial pressure. Higher mean arterial pressure and female sex were also positively associated with a change in augmentation index over time. No risk factors were identified to be associated with change in carotid radial or femoral foot pulse wave velocity. The only risk factor associated with heart rate variability was time-weighted mean hemoglobin A1c. The heart rate variability outcomes SDNN, RMSSD, and PNN50 decreased (indicating worsening) by an average of 3.8ms, 3.8ms, and 2.3%, respectively, for every 1% increase in time-weighted mean hemoglobin A1c during the 5-year follow-up (all p < 0.0001). None of the other risk factors, including age, race, sex, body mass index, smoking, or low-density lipoprotein cholesterol, were significantly associated with any of the heart rate variability indices.
Table 2.
Multivariable association of risk factors with changes in arterial stiffness outcomes during the 5-year follow-up
| Risk factor at the 5-year follow-up assessment | Pulse wave velocity carotid femoral (m/s) a |
Pulse wave velocity carotid radial (m/s) a |
Pulse wave velocity femoral foot (m/s) a |
Augmentation index (%) a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β estimate | 95% CI | P value | β estimate | 95% CI | P value | β estimate | 95% CI | P value | β estimate | 95% CI | P value | |
| Age (per year) | 0.013 | −0.080, 0.106 | 0.79 | 0.035 | − 0.081, 0.152 | 0.55 | 0.005 | −0.178, 0.188 | 0.95 | −0.115 | −0.512, 0.283 | 0.57 |
| Male | 0.110 | −0.369, 0.589 | 0.65 | −0.056 | − 0.648, 0.536 | 0.85 | −0.027 | −1.004, 0.950 | 0.95 | − 2.875 | − 5.493, −0.258 | 0.03 |
| Hispanic | 0.246 | −0.383, 0.876 | 0.44 | 0.594 | − 0.176, 1.363 | 0.13 | −0.311 | −1.004, 0.950 | 0.63 | 0.001 | −2.760, 2.761 | 0.99 |
| Non-Hispanic Black | 0.563 | −0.121, 1.247 | 0.11 | 0.288 | − 0.539, 1.115 | 0.49 | −0.428 | −1.791, 0.934 | 0.53 | 1.286 | −1.584, 4.157 | 0.38 |
| Body mass index (per 5 kg/m2) | −0.079 | −0.231, 0.073 | 0.31 | 0.005 | − 0.162, 0.172 | 0.95 | −0.134 | −0.407, 0.138 | 0.33 | −0.526 | −1.076, 0.024 | 0.06 |
| Smoking | −0.452 | −0.894, 0.001 | 0.05 | 0.121 | − 0.414, 0.656 | 0.65 | −0.243 | −1.082, 0.597 | 0.57 | 1.790 | −0.113, 3.694 | 0.06 |
| Hemoglobin A1c (per 1%)* | 0.100 | 0.007, 0.193 | 0.03 | 0.011 | − 0.106, 0.128 | 0.85 | 0.113 | −0.061, 0.288 | 0.20 | 0.002 | −0.387, 0.392 | 0.99 |
| Mean arterial pressure (per 10 mm Hg) | 0.421 | 0.223, 0.619 | <0.0001 | 0.237 | − 0.001, 0.476 | 0.05 | 0.355 | −0.043, 0.754 | 0.08 | 1.886 | 1.017, 2.755 | <0.0001 |
| Low-density lipoprotein cholesterol (per 5 mg/dL) | −0.023 | −0.053, 0.006 | 0.11 | 0.009 | − 0.026, 0.045 | 0.62 | −0.052 | −0.109, 0.005 | 0.07 | 0.003 | −0.126, 0.132 | 0.96 |
| Model R-square | 0.28 | 0.15 | 0.37 | 0.29 | ||||||||
Multivariable linear regression models include the 5-year change in the arterial stiffness assessment (dependent variable) as a function of the initial value of the outcome, height (in the augmentation index model), and heart rate (except in augmentation index model) by default (data not shown for these covariates). In addition, models included a measure of glycemic control, sex (referent group= ‘female’), race–ethnicity (referent group = ‘non-Hispanic White’), smoking during the 5-year interval (referent group= ‘no’), and the following continuous variables (per unit change as indicated) from the time of the 5-year follow-up assessment: age, body mass index, low-density lipoprotein cholesterol, and mean arterial blood pressure
Hemoglobin A1c entered in the model as a time-weighted mean
Beta coefficient (β) estimates, and associated 95% confidence intervals [CI] and P-values are provided for each covariate and significant results with P-values <0.05 are bolded
Higher value is worse
Discussion
We have previously documented worse arterial stiffness and heart rate variability in this cohort of adolescents with youth-onset type 2 diabetes compared to obese controls using data from the initial arterial stiffness and heart rate variability assessment [3, 5]. Now we report novel data that show worsening of arterial stiffness and heart rate variability as this cohort progresses into young adulthood that appears to be influenced by blood pressure and glucose control over time.
The annual change in carotid femoral pulse wave velocity observed here was 0.15m/s. This is similar to adults in their mid-1950s with 15–20 years of diabetes duration which have been shown to have a comparable annual change of carotid femoral pulse wave velocity between 0.11 and 0.20 m/s [22, 23]. Only one other study has reported change in carotid femoral pulse wave velocity over time in adolescents with type 2 diabetes where the annual change in carotid femoral pulse wave velocity was 0.08m/s [17] which was higher than the annual change of 0.04m/s observed in lean controls. Collectively, these data document rapid worsening of arterial stiffness in individuals with youth-onset type 2 diabetes during young adulthood. Differences in study populations, follow-up times, and devices used to measure arterial stiffness prevent direct comparisons of the rate of change in adults versus youth.
Overall heart rate variability (SDNN) declined and as did other heart rate variability indices indicating parasympathetic and sympathetic loss. These data suggest an increased risk for cardiac autonomic dysfunction in young adults with youth-onset type 2 diabetes. A meta-analysis of 21 studies that included nearly 3500 adults post-myocardial infarction found a SDNN of < 70ms was associated with a fourfold higher risk of death compared to a SDNN > 70ms [21]. The high percent of SDNN < 70ms at both initial and follow-up evaluations suggests this young adult cohort is at high risk for future cardiovascular complications.
We did not find major differences in the change over time in arterial stiffness and heart rate variability by sex or race–ethnicity. In unadjusted models, RMSSD, a parasympathetic component of heart rate variability, was lower or worse in non-Hispanic Blacks versus non-Hispanic Whites but this racial difference did not persist after adjusting for other risk factors. When height was added to the linear regression models, female sex was found to be associated with greater change in augmentation index over time (change in augmentation index for females was + 6.4% vs + 3.6% in males). The greater change in augmentation index among females could reflect an increased taper of the distal aorta in females versus males or may simply reflect a greater change in arterial stiffness over time. Studies of young populations generally show lower augmentation index in females versus males [4] that reverses with aging [24, 25]. Our initial cross-sectional study found higher augmentation index in females compared to males [3]. This suggests this reversal is happening at a much younger age in those with youth-onset type diabetes.
Higher cumulative glycemic exposure, represented by the time-weighted mean hemoglobin A1c, was associated with worse arterial stiffness and heart rate variability and greater progression. These results are consistent with adult data reporting associations of higher hemoglobin A1c with greater vascular stiffness [26, 27] and reduced heart rate variability [11]. These results are also concordant with our cross-sectional work using initial data in this cohort [3, 5]. Similar to hemoglobin A1c, higher blood pressure as assessed by mean arterial pressure was also found to be associated with worse carotid femoral pulse wave velocity and augmentation index as seen in the prior cross-sectional analysis of this cohort [3, 5]. In concert, these results identify two potentially modifiable risk factors, blood pressure and glycemic control, that appear important for arterial stiffness and heart rate variability and their progression over time. Whether hemoglobin A1c and blood pressure lowering improve arterial stiffness and heart rate variability in a youth-onset type 2 diabetes population has yet to be determined.
The strengths of this study include a relatively large sample size, a multi-ethnic cohort of individuals with youth-onset type 2 diabetes, and a longitudinal design over 5 years in a high-risk cohort of young adults with type 2 diabetes onset in adolescence. The results are limited by loss of sample size due to missing initial or follow-up assessments, but is one of the largest adolescent cohorts reported on to date. Additionally, we could not include some risk factors that may be important, including levels of exercise and diet, as they were not assessed close enough in time to the arterial stiffness and heart rate variability measurements.
Conclusions
In conclusion, arterial stiffness and heart rate variability continue to worsen over time and these changes are evident by late adolescence and into young adulthood in individuals with youth-onset diabetes. Treatment and interventions are needed to slow these processes to improve long-term outcomes in this high-risk population. Our results point to lowering of hemoglobin A1c and blood pressure that may improve long-term cardiovascular outcomes.
Supplementary Material
Acknowledgements
A complete list of individuals in the TODAY Study Group is presented in the Additional file: Data S3. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Funding
This work was completed with funding from NIDDK and the NIH Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIDDK project office was involved in all aspects of the study, including: design and conduct; collection, management, analysis, and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- PNN50
percent of adjacent NN intervals with a difference greater than 50msec
- RMSSD
root-mean-square differences of successive NN intervals
- SDNN
SD of the R–R interval (termed NN interval)
- TODAY
Treatment Options for type 2 Diabetes in Adolescents and Youth
Footnotes
Trial Registration: ClinicalTrials.gov NCT00081328
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00592–021-01796–6.
Availability of data and materials All data and materials are available upon request.
Declarations
Conflict of interest The authors declare that they have no competing interests to report.
Consent for publication The authors consent to the publication of this article.
Ethical approval The study was approved by institutional review boards at all 15 centers and all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent All participants and guardians provided written informed assent and/or consent as appropriate for age and local guidelines.
References
- 1.Dabelea D, Stafford JM, Mayer-Davis EJ et al. (2017) Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA J Am Med Assoc 317(8):825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal M, Divers J, Urbina EM et al. (2018) Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth Cohort Study. Pediatric Diabetes 19(4):680–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah AS, El Ghormli L, Gidding SS et al. (2018) Prevalence of arterial stiffness in adolescents with type 2 diabetes in the TODAY cohort: Relationships to glycemic control and other risk factors. J Diabetes Complicat 32(8):740–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM (2010) Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens 28(8):1692–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AS, El Ghormli L, Vajravelu ME et al. (2019) Heart rate variability and cardiac autonomic dysfunction: prevalence, risk factors, and relationship to arterial stiffness in the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care 42(11):2143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55(13):1318–27 [DOI] [PubMed] [Google Scholar]
- 7.Urbina EM, Williams RV, Alpert BS et al. (2009) Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 54(5):919–50 [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Maser RE, Mitchell BD, Freeman R (2003) Diabetic autonomic neuropathy. Diabetes Care 26(5):1553–79 [DOI] [PubMed] [Google Scholar]
- 9.Maser RE, Mitchell BD, Vinik AI, Freeman R (2003) The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 26(6):1895–901 [DOI] [PubMed] [Google Scholar]
- 10.Pop-Busui R, Boulton AJ, Feldman EL et al. (2017) Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 40(1):136–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benichou T, Pereira B, Mermillod M et al. (2018) Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PloS One 13(4):e0195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha SA, Park YM, Yun JS et al. (2018) Time- and frequency-domain measures of heart rate variability predict cardiovascular outcome in patients with type 2 diabetes. Diabetes Res Clin Pract 143:159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal M, Urbina EM, Wadwa RP et al. (2013) Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care 36(8):2351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AS, Jaiswal M, Dabelea D et al. (2020) Cardiovascular risk and heart rate variability in young adults with type 2 diabetes and arterial stiffness: the SEARCH for Diabetes in Youth Study. J Diabetes Complicat 34(10):107676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell GF, Hwang SJ, Vasan RS et al. (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121(4):505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuoka O, Otsuka K, Murakami S et al. (2005) Arterial stiffness independently predicts cardiovascular events in an elderly community: Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother 59(Suppl 1):S40–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryder JR, Northrop E, Rudser KD et al. (2020) Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J Am Heart Assoc 9(10):e014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeitler P, Epstein L, Grey M et al. (2007) Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatric Diabetes 8(2):74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitler P, Hirst K, Pyle L et al. (2012) A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366(24):2247–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TODAY Study Group, Bjornstad P, Drews KL, et al. (2021) Long-term complications in youth-onset type 2 diabetes. N Engl J Med 385(5):416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, Levy D (1996) Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94(11):2850–5 [DOI] [PubMed] [Google Scholar]
- 22.Ferreira MT, Leite NC, Cardoso CR, Salles GF (2015) Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care 38(5):897–904 [DOI] [PubMed] [Google Scholar]
- 23.Moh MC, Sum CF, Tavintharan S et al. (2017) Baseline predictors of aortic stiffness progression among multi-ethnic Asians with type 2 diabetes. Atherosclerosis 260:102–9 [DOI] [PubMed] [Google Scholar]
- 24.Chester R, Sander G, Fernandez C, Chen W, Berenson G, Giles T (2013) Women have significantly greater difference between central and peripheral arterial pressure compared with men: the Bogalusa Heart Study. J Am Soc Hypertens 7(5):379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogola BO, Zimmerman MA, Clark GL et al. (2018) New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol 315(5):H1073–H87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, Dolan LM, Kimball TR et al. (2009) Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and young adults with type 2 diabetes mellitus. J Clin Endocrinol Metab 94(10):3740–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotb NA, Gaber R, Salama M, Nagy HM, Elhendy A (2012) Clinical and biochemical predictors of increased carotid intimamedia thickness in overweight and obese adolescents with type 2 diabetes. Diab Vasc Dis Res 9(1):35–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


