Fig. 3. Mechanism of GSDM pore formation.
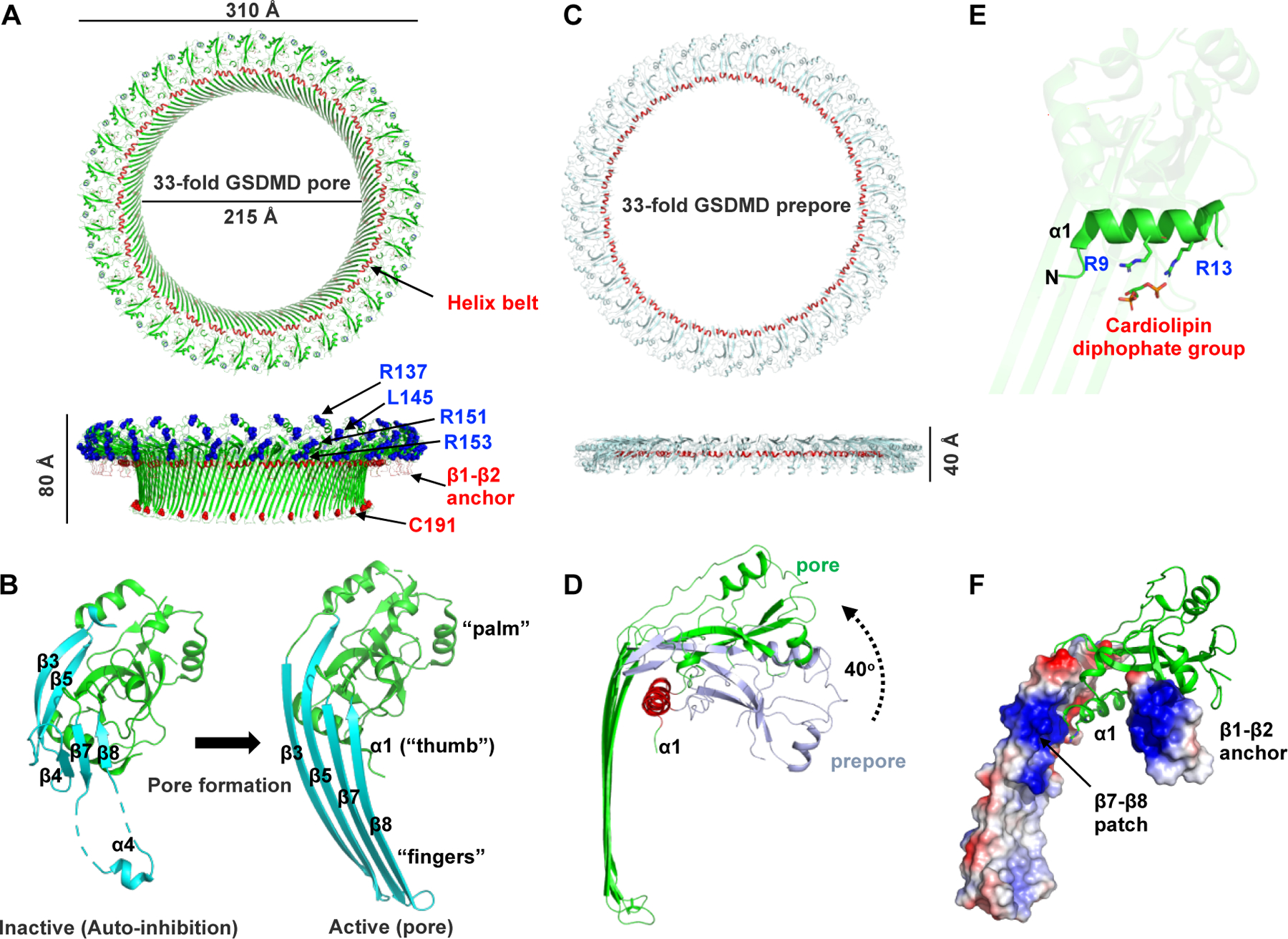
(A) Cryo-EM structure of the 33-subunit human GSDMD pore (PDB: 6VFE). The pore features a large transmembrane β-barrel and a globular domain on the cytosolic side. The “helix belt” and the β1–β2 anchor that binds to the plasma membrane are labeled and colored red. A previously reported “four-basic-residue” patch is also labeled and colored blue. Cys191 residue that are modified by disulfiram (DSF) are labeled. (B) Conformation change of GSDMD subunit upon pore formation. The pore forms a structure that resembles a human left hand, with the globular domain as the ‘palm’, the α1 helix as the ‘thumb’, and the membrane-inserted β-hairpins as the ‘fingers’. (C) Cryo-EM structure of the 33-subunit human GSDMD prepore. (D) Prepore-to-pore transition of a GSDMD subunit. The globular domain rotates away from the membrane by approximately 40° upon membrane insertion. (E) The two conserved basic residues in the α1 helix interact with the cardiolipin diphosphate group. (F) GSDMD pore subunit with the β1–β2 anchor and the additional β7–β8 patch is shown in the local electrostatic surface.
