Abstract
Background and Aims:
Aging exacerbates liver neutrophil infiltration and alcohol-associated liver disease (ALD) in mice and humans, but the underlying mechanisms remain obscure. This study aimed to examine the effect of aging and alcohol consumption on neutrophilic Sirtuin 1 (SIRT1) and micro-RNA-223 (miR-223), and their contribution to ALD pathogeneses.
Approach and Results:
Young and aged myeloid-specific Sirt1 knockout mice were subjected to chronic-plus-binge ethanol feeding. Blood samples from healthy controls and patients with chronic alcohol drinking who presented with acute intoxication were analyzed. Neutrophilic Sirt1 and miR-223 expression were down-regulated in aged mice compared with young mice. Deletion of the Sirt1 gene in myeloid cells including neutrophils exacerbated chronic-plus-binge ethanol-induced liver injury and inflammation and down-regulated neutrophilic miR-223 expression. Immunoprecipitation experiments revealed that SIRT1 promoted C/EBPα deacetylation by directly interacting with C/EBPα, a key transcription factor that controls miR-223 biogenesis, and subsequently elevated miR-223 expression in neutrophils. Importantly, down-regulation of SIRT1 and miR-223 expression was also observed in circulating neutrophils from middle-aged and elderly subjects compared with those from young individuals. Chronic alcohol users with acute intoxication had a reduction in neutrophilic SIRT1 expression in young and middle-aged patients, with a greater reduction in the latter group. The neutrophilic SIRT1 expression correlated with neutrophilic miR-223 and serum alanine transaminase levels in those patients.
Conclusions:
Aging increases the susceptibility of alcohol-induced liver injury in mice and humans through the down-regulation of the neutrophilic SIRT1-C/EBPα-miR-223 axis, which could be a therapeutic target for the prevention and/or treatment of ALD.
INTRODUCTION
Alcohol-associated liver disease (ALD), a major cause of morbidity and mortality worldwide; encompasses a spectrum of hepatic manifestations ranging from simple steatosis to steatohepatitis, cirrhosis, and HCC.[1–4] A wide variety of host and environmental factors and comorbidities have been shown to modify the development and progression of ALD, including age, sex, genetic factors, drinking pattern, obesity, and chronic viral hepatitis.[3,5] Among these risk factors, aging is a known risk factor for various types of chronic liver diseases including ALD.[6–8] Aged individuals tend to develop a chronic low-grade proinflammatory state as evidenced by elevated circulating levels of proinflammatory cytokines and local infiltration of inflammatory cells such as neutrophils.[9,10] Interestingly, neutrophil infiltration is also a hallmark of alcohol-associated steatohepatitis in patients and mice with chronic-plus-binge ethanol intake.[11–13] Accumulating evidence suggests that hepatic neutrophil infiltration plays an important role in promoting ALD progression, possibly by killing hepatocytes through the production of reactive oxygen species (ROS) and inflammatory mediators that promote liver inflammation and fibrosis.[11–13] However, neutrophils may also play a beneficial role in ameliorating steatohepatitis by releasing the anti-inflammatory miR-223.[14–16] In addition, neutrophil life span, recruitment, and functions are known to be regulated by both aging and alcohol consumption.[17] However, how such regulation during aging affects ALD pathogenesis remains elusive.
Sirtuin 1 (SIRT1), an NAD+-dependent histone deacetylase, is ubiquitously expressed and plays crucial roles in regulating aging-related processes, lipid metabolism, and inflammation by modulating acetylation of a wide variety of transcription factors and signaling proteins.[18–21] For example, SIRT1 was found to modulate inflammatory responses by deacetylating histones and critical transcription factors such as NF-κB p65, Forkhead box O3, and activator protein 1.[22,23] Myeloid cell SIRT1 has been reported to act as an anti-inflammatory molecule by inhibiting NF-κB-mediated transcriptional activation of proinflammatory genes.[24] In the liver, activation of SIRT1 by resveratrol (RSV) treatment has been shown to protect against high-fat diet–induced and alcohol-induced fatty liver diseases,[25,26] whereas deletion of the Sirt1 gene in hepatocytes aggravates ALD.[27] In contrast, deletion of the Sirt1 gene in intestinal epithelium protects against ALD.[28] We have previously demonstrated that SIRT1 expression in hepatocytes and hepatic stellate cells (HSCs) is down-regulated in aged mice and such down-regulation probably contributes to an increase in ALD susceptibility in these mice.[8] Nevertheless, the molecular mechanisms by which neutrophilic SIRT1 and aging modulate ALD remain ill-defined.[29]
In the current study, we demonstrated that neutrophilic SIRT1 expression was significantly down-regulated in aged mice and ethanol-fed mice. Deletion of the Sirt1 gene in myeloid cells, including neutrophils, exacerbated chronic-plus-binge ethanol-induced ALD. Mechanistically, neutrophilic SIRT1 down-regulation during aging and after alcohol feeding reduces the expression of the anti-inflammatory and antifibrotic microRNA-223 (miR-223),[14–16] ultimately resulting in the increased susceptibility to liver injury. Furthermore, we found that aging-mediated or alcohol-mediated SIRT1 down-regulation in neutrophils promoted the acetylation of CCAAT/enhancer-binding protein α (C/EBPα), a transcription factor that controls miR-223 biogenesis,[30] ultimately leading to neutrophilic miR-223 down-regulation during aging or after ethanol feeding. Finally, we found that neutrophilic SIRT1 mRNA and miR-223 were also down-regulated in middle-aged and aged humans and that acute-on-chronic drinking attenuated neutrophilic SIRT1 in young and middle-aged individuals.
MATERIALS AND METHODS
Mice and chronic-plus-binge ethanol feeding protocol
Aged C57BL/6 mice (20–24 months) were obtained from the National Institute of Aging, NIH. Myeloid cell-specific Sirt1 knockout (Sirt1Mye−/−) mice and littermate control (Sirt1f/f) mice were described previously.[24] The chronic-plus-binge ethanol feeding model was described previously.[31] All animal experiments were approved by the NIAAA Animal Care and Use Committee.
Analysis of bio-samples from healthy controls and patients with acute alcohol intoxication
We analyzed baseline laboratory data and blood samples which were collected as part of routine clinical care and authorized for research studies of healthy controls and patients with acute alcohol intoxication. Healthy controls were those without underlying medical illnesses who were seen at the Medical Examination Center, First Hospital of Anhui Medical University (AMU) (Anhui, China). Patients with alcohol use disorder with a history of chronic alcohol drinking presented with acute intoxication were those who received medical care at the Emergency Department, First Hospital of AMU. To be eligible, we excluded samples from patients with underlying viral hepatitis or other liver diseases. The following three cohorts were analyzed: Circulating neutrophils were collected from 36 healthy controls (Table S1). Sera were collected from 146 healthy controls (Table S2). Sera and circulating neutrophils were collected from 29 healthy controls and 32 patients who were intoxicated (Table S3). The study protocol and the use of bio-samples for research were approved by the Ethics Committee of the First Hospital of AMU, and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from all subjects or their relatives.
Statistical analyses
The results are expressed as the mean ± SEM. All statistical analyses were performed using GraphPad Prism software (v. 7.0a). To compare values obtained from two groups, Student t test was performed. Data from multiple groups were compared with one-way ANOVA followed by Tukey’s post-test as appropriate. The linear regression analysis was performed to determine the association between two continuous variables. p values of <0.05 were considered significant.
Other methods are described in Supporting Information.
RESULTS
Neutrophilic Sirt1 levels are decreased in aged mice and deletion of neutrophilic Sirt1 exacerbates chronic-plus-binge ethanol-induced ALD
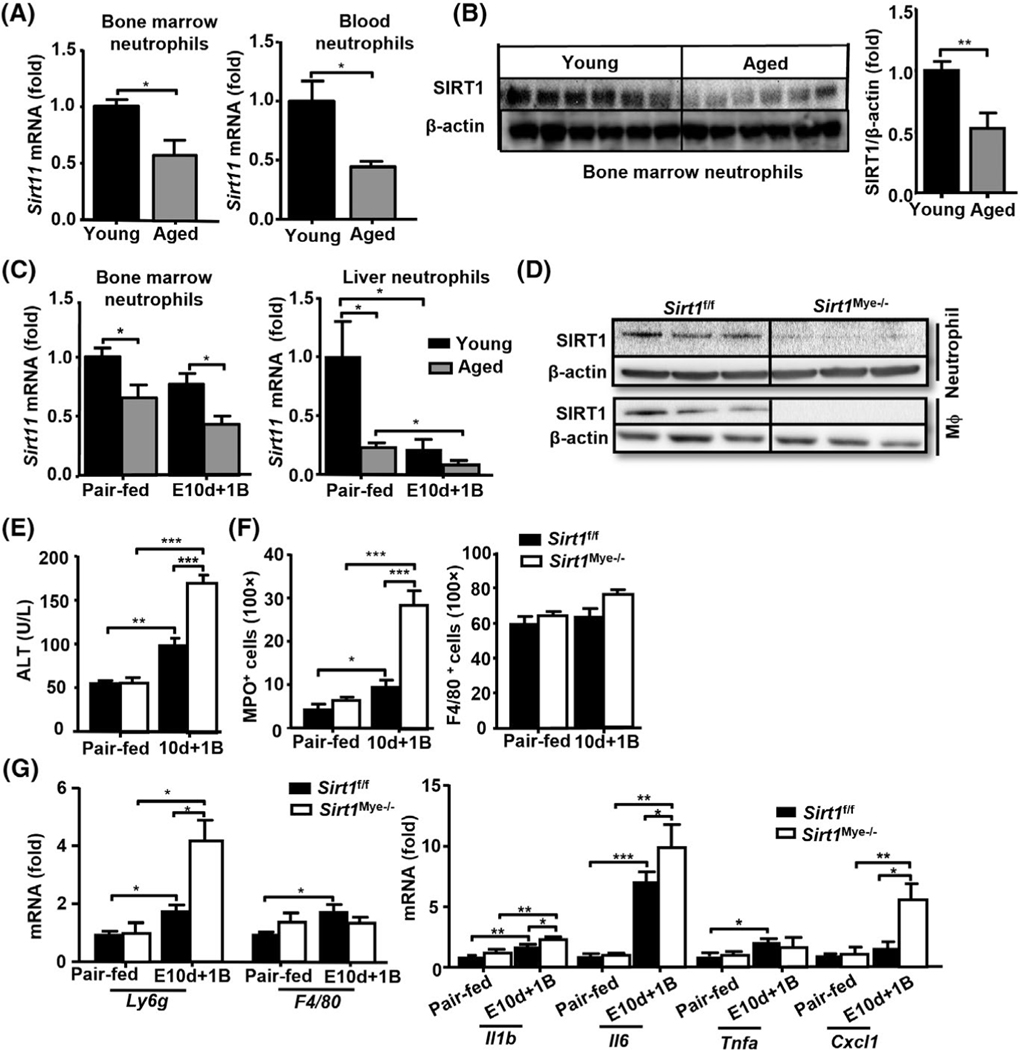
Neutrophil infiltration is a key player in the pathogenesis of ALD.[11–13] However, how neutrophils are regulated during aging and their contribution to ALD remain unclear. Our previous study reported that aged mice were more susceptible to alcohol-induced liver injury and fibrosis, which is partially due to the down-regulation of SIRT1 in hepatocytes and HSCs.[8] Thus, we wonder whether SIRT1 is also deregulated in neutrophils in aged mice. To answer this question, we first demonstrated that aged mice showed lower Sirt1 mRNA in neutrophils from both bone marrow (BM) and peripheral blood and lower SIRT1 protein levels in BM neutrophils compared with young mice (Figure 1A,B). To better understand the regulation of neutrophilic SIRT1 in ALD, aged (22–24 months) and young male mice (6–8 weeks) were subjected to chronic-plus-binge ethanol (E10+1B) challenge. As illustrated in Figure 1C, Sirt1 mRNA expression in liver neutrophils was significantly decreased in E10d+1B-fed mice compared with pair-fed mice in young or aged mice, whereas Sirt1 mRNA in BM neutrophils showed a decreasing trend after alcohol feeding. Moreover, aged male mice showed higher levels of serum alanine aminotransferase (ALT), white blood cells, and circulating monocytes than that of young mice in both pair-fed and ethanol-fed groups (Figure S1A–C).
FIGURE 1.
Neutrophilic SIRT1 levels are deceased in aged mice and deletion of Sirt1 in myeloid cells exacerbates chronic-plus-binge ethanol-induced liver injury. (A,B) Neutrophils were isolated from BM and blood of young (n = 6) and aged (n = 6) mice. Expressions of Sirt1 mRNA and protein were measured by RT-qPCR (A) and Western blotting (B), respectively. Quantification of proteins is shown in the right panel B. (C) Young (6–8 weeks) and aged C57BL/6J mice (24 months) were subjected to pair-fed or 10 day-plus-one binge (E10d+1B) ethanol feeding (n = 5 to 6 in each group). Mice were euthanized 9 h after gavage. BM and liver neutrophils were collected from pair-fed and EtOH-fed young or aged mice. Sirt1 mRNA levels were measured by RT-qPCR. (D) Western blot analysis of SIRT1 protein levels from BM neutrophils and peritoneal macrophages in Sirt1Mye−/− and littermate control (Sirt1f/f) mice. (E–G) Sirt1Mye−/− (n = 7) and Sirt1f/f (n = 9) were subjected to pair-fed or E10d+1B ethanol feeding. Mice were euthanized 9 h after gavage. Serum ALT levels were measured (E). Liver tissues were subjected to immunostaining with anti-MPO and anti-F4/80 staining, and the number of MPO+ neutrophils and F4/80+ macrophages were quantified (F). RT-qPCR analyses of liver Ly6g and F4/80 mRNA (G, left). RT-qPCR analyses of liver inflammatory cytokines and chemokines mRNA (G, right). Values represent means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
To investigate the functions of neutrophilic SIRT1 in ALD, we generated myeloid cell-specific Sirt1 knockout mice (Sirt1Mye−/−) and littermate control mice (Sirt1f/f) and challenged them with E10d+1B feeding. Deletion of SIRT1 protein in myeloid cells from Sirt1Mye−/− mice was demonstrated by western blot analyses (Figure 1D). Sirt1Mye−/− mice had higher levels of serum ALT, greater steatosis, and more myeloperoxidase (MPO)+ neutrophil infiltration in the liver after ethanol feeding than that in Sirt1f/f mice (Figures 1E,F and S2A). The number of circulating neutrophils was significantly elevated in both Sirt1f/f and Sirt1Mye−/− mice after ethanol feeding, but there were no differences in the number of white blood cells and circulating neutrophils between these two groups (Figure S2B). In addition, hepatic expression of Ly6g (a marker of neutrophils) was higher in Sirt1Mye−/− mice versus Sirt1f/f mice (Figure 1G, left), whereas the number of hepatic F4/80+ macrophages was comparable between these two groups (Figures 1F,G and S2A). Next, we also examined hepatic mRNA expression of several proinflammatory mediators and found that hepatic expressions of Il-1b, Il-6, and chemokine (C-X-C motif) ligand 1 were elevated in both ethanol-fed Sirt1f/f and Sirt1Mye−/− mice with higher levels in the latter group; whereas the expression of several other mediators was comparable between these two groups (Figures 1G and S2C).
To exclude the possibility that deletion of the Sirt1 gene may alter ethanol metabolism and subsequently affect the susceptibility to ethanol-induced liver injury,[32] we examined different ethanol metabolizing enzymes in liver tissues. As shown in Figure S2D, there were no differences in the expression of hepatic CYP2E1, alcohol dehydrogenase 1 (ADH1) and acetaldehyde dehydrogenase 2 (ALDH2) between the Sirt1f/f and Sirt1Mye−/−groups. Moreover, the protein levels of these enzymes in liver tissues were comparable between aged and young mice (Figure S2E).
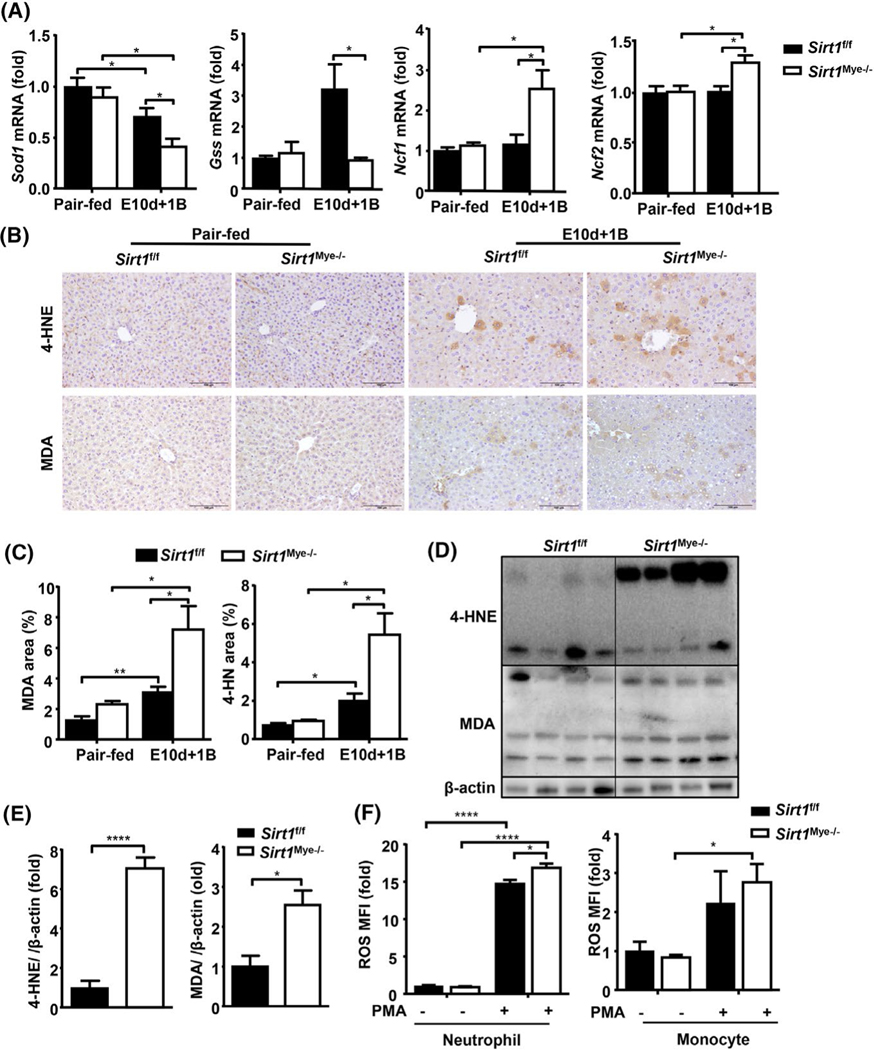
Oxidative stress and inflammation have been considered as two key features for ALD.[5] Therefore, we measured several ROS-related genes, such as glutathione synthetase (Gss), superoxide dismutase (Sod) 2, neutrophil cytosolic factor 1 (Ncf) 1, and Ncf2. As shown in Figure 2A, the expression of antioxidant genes (e.g., Sod1 and Gss) was remarkably decreased, whereas the expression of prooxidant genes (e.g., Ncf1 and Ncf2) was increased in Sirt1Mye−/− compared with Sirt1f/f mice after E10d+1B feeding. Hepatic levels of lipid peroxide including malonaldehyde (MDA) and 4-hydroxynonenal (4-HNE) were elevated in both ethanol-fed Sirt1f/f and Sirt1Mye−/− mice, with a greater increase in Sirt1Mye−/− mice (Figure 2B–E). In in vitro experiments, we observed that PMA treatment markedly increased ROS production in BM neutrophils and monocytes from Sirt1f/f and Sirt1Mye−/− mice, with higher ROS levels in neutrophils from Sirt1Mye−/− mice than those from Sirt1f/f mice. There were no differences in ROS production in PMA-treated monocytes between Sirt1f/f and Sirt1Mye−/− mice (Figure 2F).
FIGURE 2.
Sirt1Mye−/− mice are more susceptible to chronic-plus-binge ethanol-induced oxidative stress and inflammation in the liver. Sirt1Mye−/− (n = 7) and Sirt1f/f (n = 9) mice were subjected to E10d+1B or pair feeding. Mice were euthanized 9 h after gavage. Liver samples were collected and analyzed. (A) RT-qPCR analysis of reactive oxgen species (ROS)-related genes. (B,C) Liver tissues were subjected to immunostaining with an anti-malonaldehyde (MDA) or anti-4-HNE antibody. Representative images are shown in panel B. Relative staining is quantified and shown in panel C. (D,E) Western blot analyses of 4-HNE and MDA, and their quantitation results are shown in panel E. (F) BM neutrophils from Sirt1Mye−/− and Sirt1f/f were isolated and stimulated with or without phorbol 12-myristate 13-acetate (PMA). The ROS levels were measured by flow cytometric analyses. The ROS levels are calculated and shown. MFI relative fluorescence unit. Values represent means ± SEM. *p < 0.05; **p < 0.01; ****p < 0.0001
SIRT1 regulates neutrophil-specific miR-223 in aged mice
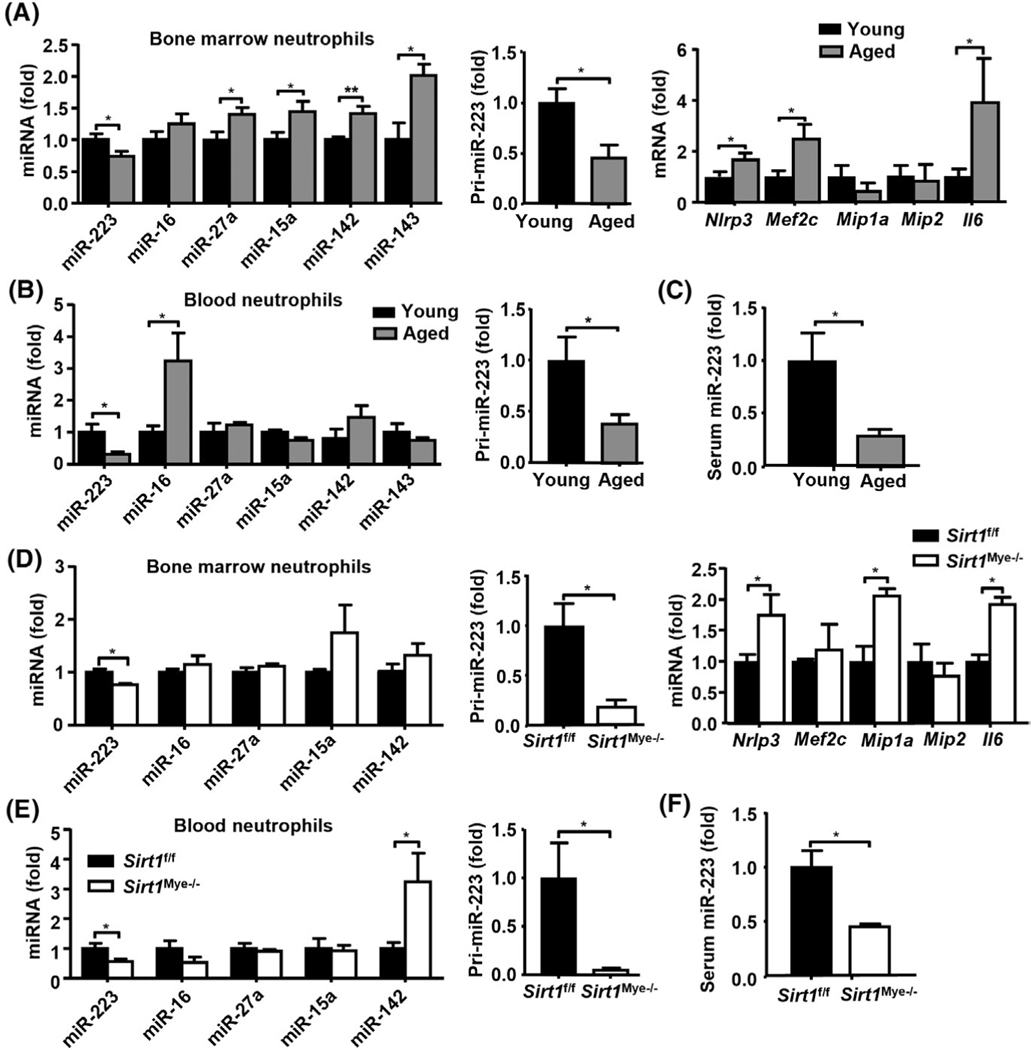
To understand how SIRT1 regulates ALD in aged mice, we examined whether SIRT1 regulates miRNAs in neutrophils given that miRNAs play an important role in aging processes[33] and ALD pathogenesis.[34] First, we examined the expression of the five most abundant miRNAs in neutrophils isolated from young and aged mice, including miR-223, miR-16, miR-27a, miR-15a, and miR-142.[35] As shown in Figure 3A (left panel), a significant down-regulation of miR-223 in BM neutrophils from aged mice was noted, whereas the levels of other miRNAs were higher in neutrophils from aged mice compared with young mice. Furthermore, primary miR-223 (pri-miR-223) levels were also down-regulated in BM neutrophils from aged mice (Figure 3A, middle panel), suggesting that transcriptional regulation may be involved in the down-regulation of miR-223 expression in neutrophils during aging. Next, we measured several proven target genes of miR-223 that are involved in neutrophilic chemotaxis and activation,[15,36] and found that NOD-, LRR-, and pyrin domain-containing protein 3 (Nlrp3), Mef2c, and Il-6 mRNA levels were elevated in BM neutrophils from aged mice compared with young mice (Figure 3A, right panel). The significant down-regulation of miR-223 and pri-miR-223 was also observed in peripheral neutrophils from aged mice compared with those from young mice (Figure 3B). Consistently, aged mice had a much lower serum level of miR-223 than young mice (Figure 3C).
FIGURE 3.
Neutrophil-specific miR-223 is down-regulated in neutrophils from aged mice and Sirt1Mye−/− mice. (A–C) BM neutrophils, blood neutrophils, and serum were collected from young (6–8 weeks, n = 6) and aged (22–24 months, n = 6) mice. RT-qPCR analyses of neutrophil-specific miRNAs, pri-miR-223, and miR-223 target genes in BM neutrophils (A). RT-qPCR analyses of neutrophil-specific miRNAs and pri-miR-223 in peripheral blood neutrophils (B). Serum miR-223 expression was measured (C). (D–F) BM neutrophils, blood neutrophils, and serum were collected from Sirt1Mye−/− and Sirt1f/f mice (n = 6). RT-qPCR analyses of neutrophil-specific miRNAs, pri-miR-223, and miR-223 target genes in BM neutrophils (D). RT-qPCR analyses of neutrophil-specific miRNAs and pri-miR-223 in peripheral blood neutrophils (E). Serum miR-223 expression was analyzed (F). Values represent means ± SEM. *p < 0.05, **p < 0.01
To examine whether SIRT1 directly regulates neutrophilic miR-223 expression, we measured miR-223 in Sirt1Mye−/− mice and found that Sirt1Mye−/− mice showed lower levels of miR-223 but no other tested miRNAs in BM neutrophils compared with Sirt1f/f mice (Figure 3D). Pri-miR-223 levels and miR-223 target genes Nlrp3, Mip1a, and Il-6 mRNA levels were higher in neutrophils from Sirt1Mye−/− mice than those from Sirt1f/f mice (Figure 3D). Moreover, miR-223 and pri-miR-223 expression in circulating neutrophils, and serum miR-223 levels were lower in Sirt1Mye−/− mice than in Sirt1f/f mice (Figure 3E,F). Interestingly, no differences in Sirt1 mRNA and SIRT1 protein levels were observed in BM neutrophils between miR-223−/− and WT mice (Figure S3A,B). Collectively, SIRT1 regulates miR-223 in neutrophils but not vice versa.
Regulation of neutrophilic miR-223 during the aging process and ethanol feeding: A potential role of SIRT1
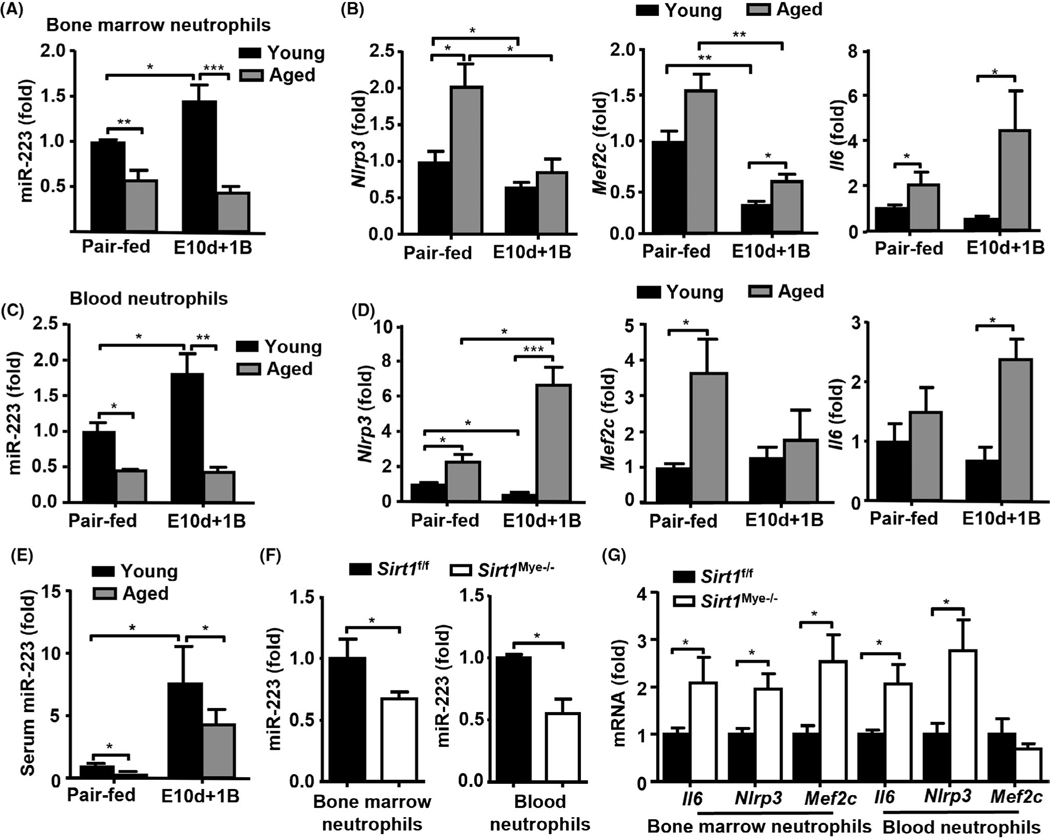
To determine how neutrophilic miR-223 is involved in aging and ALD, we fed aged and young male mice with E10d+1B challenge and found that ethanol feeding elevated miR-223 in BM neutrophils in young mice, but such elevation was completely blunted in neutrophils from aged mice (Figure 4A). The expression of miR-223 target genes (i.e., Nlrp3, Mef2c, and Il-6) in BM neutrophils from aged mice was higher than that in BM neutrophils from young mice (Figure 4B). Similar results were observed in blood neutrophils isolated from both pair-fed and ethanol-fed aged and young mice (Figure 4C,D). Interestingly, alcohol feeding elevated serum miR-223 levels in young mice, which was consistent with our previous results,[36] whereas serum miR-223 levels were significantly lower in ethanol-fed aged mice compared with those in ethanol-fed young mice (Figure 4E).
FIGURE 4.
Regulation of miR-223 levels in serum and neutrophils from young and aged mice after ethanol feeding. (A–E) Young C57BL/6J (6–8 weeks) and aged C57BL/6J mice (24 months) were subjected to E10d+1B or pair feeding (n = 8–9 in each group), and mice were euthanized 9 h after gavage. BM, blood neutrophils, and serum were collected for miR-223 measurement. RT-qPCR analyses of miR-223 (A, C) and its target genes (B, D) in neutrophils, and serum miR-223 (E). (F-G) Sirt1Mye−/− and Sirt1f/f mice were subjected to E10d+1B or pair feeding (n = 5–7 in each group). RT-qPCR analyses of miR-223 (F) and its target genes (G) in neutrophils. Values represent means ± SEM. *p < 0.05; **p < 0.01
To address whether the neutrophilic miR-223 expression is controlled by SIRT1, we measured the expressions of miR-223 and its target genes in neutrophils from ethanol-fed mice. As illustrated in Figure 4F,G, in both BM and blood neutrophils, the expression of miR-223 was lower, whereas the levels of its target genes (i.e., Nlrp3, Mef2c, and Il-6) were higher in ethanol-fed Sirt1Mye−/− mice than those in ethanol-fed Sirt1f/f mice, except that the expression of Mef2c in blood neutrophils was comparable in these two groups of mice.
SIRT1 up-regulates neutrophilic miR-223 expression by deacetylating C/EBPα, which is suppressed during aging
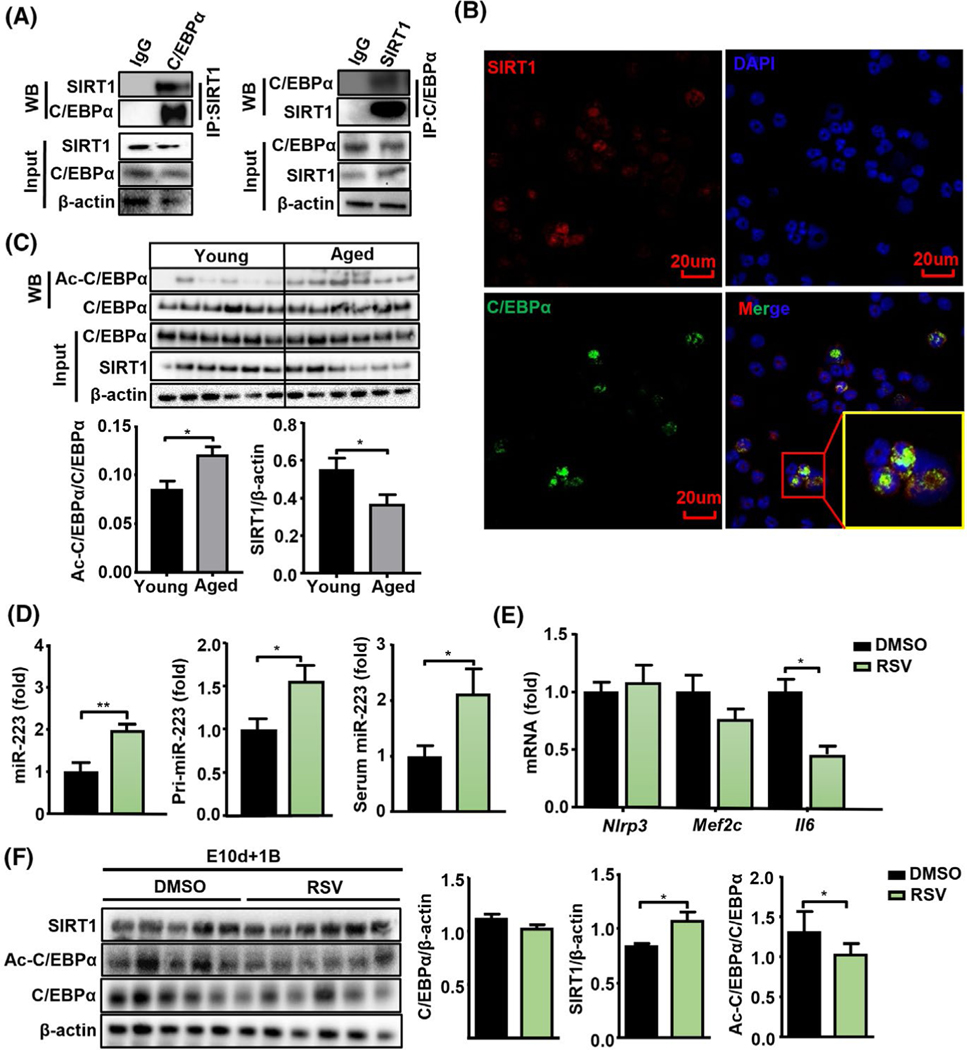
Next, we examined how SIRT1 regulates neutrophilic miR-223 expression during aging. The mRNA levels of many key genes involved in miRNA transcription and biogenesis[37] were comparable in neutrophils from aged and young mice or human subjects (Figure S4A,B and Table S1), suggesting that miR-223 dysregulation during aging may occur at pretranscriptional levels. SIRT1 is a well-known histone and protein deacetylase to regulate C/EBPα expression,[38] and C/EBPα is well-documented to control miR-223 expression in neutrophils,[30] thus we hypothesized that SIRT1 may regulate miR-223 expression through C/EBPα deacetylation. To address this question, we examined the interaction between SIRT1 and C/EBPα by co-immunoprecipitation assay. As shown in Figure 5A, SIRT1 immunoprecipitated with C/EBPα in neutrophils; meanwhile, reciprocal IP with C/EBPα antibodies showed that C/EBPα also immunoprecipitated with SIRT1. Colocalization of SIRT1 and C/EBPα was detected in the nuclei of neutrophils by confocal microscopy (Figure 5B). To assess the acetylation status of C/EBPα during aging, neutrophils from ethanol-fed young and aged mice were subjected to detection of C/EBPα acetylation. We found that acetylated C/EBPα levels were higher, whereas SIRT1 levels were lower in neutrophils from ethanol-fed aged mice compared with those from ethanol-fed young mice; total C/EBPα protein expression was not altered between these two groups (Figure 5C). In addition, acetylation levels of p53 and the p65 subunit of NF-κ B, two vital nonhistone deacetylation targets of SIRT1, were slightly increased in neutrophils from ethanol-fed aged mice but this increase did not reach statistical significance (Figure S4C).
FIGURE 5.
Acetylated C/EBPα in neutrophils are elevated in aged mice but decreased in SIRT1 agonist-treated mice. (A) Immunoprecipitation (IP) analysis of the interaction of C/EBPα and SIRT1. BM neutrophil cell lysates were immunoprecipitated with an anti-SIRT1 or anti-C/EBPα. Then the precipitates and cell lysates (input) were immunoblotted with an anti-C/EBPα antibody or anti-SIRT1 antibody as indicated. (B) Immunostaining analysis of the colocalization of SIRT1 (red) and C/EBPα (green) in neutrophils. DAPI staining was used to locate the nucleus. The scale bars represent 20 μm. (C) BM neutrophils were collected from ethanol-fed young and aged mice and subjected to western blot analyses of SIRT1 and C/EBPα acetylation levels (Figure 5C, upper). Acetylated C/EBPα and SIRT1 levels were quantified (n = 6) (Figure 5C, lower). (D–F) Aged mice (19–20 months, n = 5–6 in each group) were subjected to E10d+1B feeding with or without RSV treatment (100 mg/kg/day), then the mice were euthanized 9 h after gavage and neutrophils were isolated from BMs. Neutrophilic miR-223 and pri-miR-223, and serum miR-223 were measured (D). RT-qPCR analyses of miR-223 target genes (Nlrp3, Mef2c, and Il-6) in neutrophils (E). Western blot analyses of SIRT1 and C/EBPα acetylation levels in neutrophils (F). Quantitation of proteins is shown. Values represent means ± SEM. *p < 0.05
Furthermore, we examined whether RSV, a SIRT1 agonist that has a hepatoprotective effect,[39] can up-regulate neutrophil-specific miR-223 expression and attenuate ALD by treating ethanol-fed aged mice with RSV. Our results revealed that RSV treatment significantly reduced serum ALT levels, improved hepatic steatosis, and decreased hepatic MPO+ neutrophil infiltration without affecting the number of hepatic F4/80+ macrophages in these mice (Figure S5A–D). Furtherly, ROS production (4-HNE and MDA) in RSV treated liver tissues was significantly lower than that in the control group, suggesting that RSV treatment attenuates ROS production (Figure S5E). Moreover, RSV treatment also elevated expression of miR-223 and pri-miR-223 in BM neutrophils, and serum miR-223 levels (Figure 5D) but down-regulated the expression of Il-6, one of the miR-223 target genes in neutrophils (Figure 5E). RSV treatment markedly reduced acetylated C/EBPα levels without affecting total C/EBPα in neutrophils (Figure 5F).
SIRT1 interacts with C/EBPα and induces its deacetylation, eventually elevating miR-223 expression in vitro
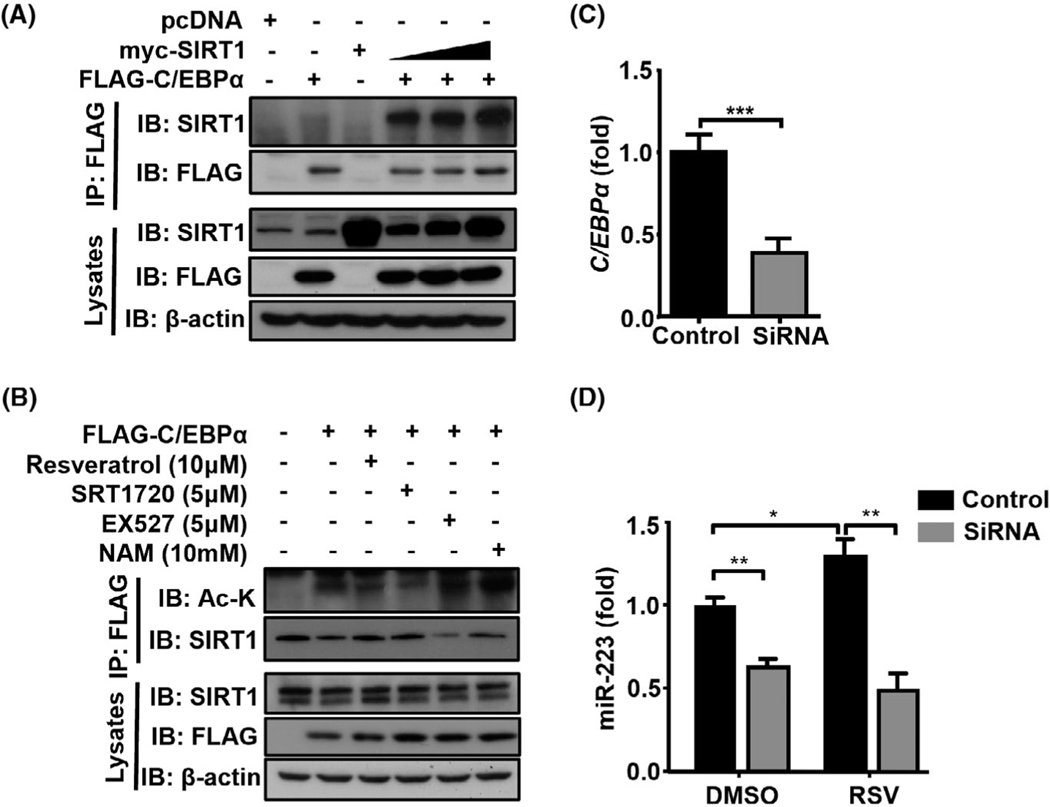
To further study whether SIRT1 and C/EBPα physically interact with each other, we co-transfected the tagged SIRT1 and C/EBPα constructs in HEK293T cells and performed an immunoprecipitation assay. Immunoprecipitation of FLAG-C/EBPα with anti-FLAG co-i mmunoprecipitated SIRT1 (Figure 6A) further confirmed that SIRT1 interacts with C/EBPα. Next, we found that treatment with the SIRT1 agonists (RSV or SRT1720) reduced C/EBPα acetylation, whereas treatment with SIRT inhibitors (EX527 or NAM) enhanced it (Figure 6B), suggesting that SIRT1 regulates epigenetic modulation of C/EBPα.
FIGURE 6.
SIRT1 interacts with C/EBPα and leads to its deacetylation, thereby inducing miR-223 expression. (A) Plasmids encoding myc-tagged SIRT1 were co-transfected with FLAG-tagged C/EBPα into HEK293T cells. Cell lysates were immunoprecipitated with an anti-FLAG antibody. The precipitates and lysates were immunoblotted with different antibodies as indicated. (B) HEK293T cells were transfected with plasmids encoding FLAG-tagged C/EBPα and then treated with RSV, SRT1720, EX527, or NAM for 24 h. Cell lysates were subjected to immunoprecipitation with an anti-FLAG antibody, followed by immunoblotting with antiacetylated lysine to detect C/EBPα acetylation. (C) HL-60 cells were treated with C/ebpa siRNA or control siRNA, followed by RT-qPCR analyses of C/ebpa expression. (D) HL-60 cells were treated with C/ebpa siRNA or control siRNA, followed by treatment with RSV (10 μM) or the vehicle (DMSO) for 6 h. Expression of miR-223 was measured by RT-qPCR. Values represent means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
Next, we examined whether SIRT1 promotes miR-223 expression through the regulation of C/EBPα, we knocked down CEBPa expression using CEBPa siRNA. Knockdown of CEBPa was confirmed by decreased its mRNA expression (Figure 6C). Knockdown of CEBPa reduced miR-223 expression in HL-60 cells in the DMSO control group (Figure 6D). Treatment with the SIRT1 activator RSV enhanced the expression of miR-223 in the control group, such elevation was disappeared in the CEBPa siRNA group (Figure 6D), suggesting that C/EBPα plays a crucial role in SIRT1-mediated induction of miR-223 levels in neutrophil-like HL-60 cells.
Down-regulation of circulating miR-223 and neutrophilic SIRT1 mRNA and miR-223 during aging
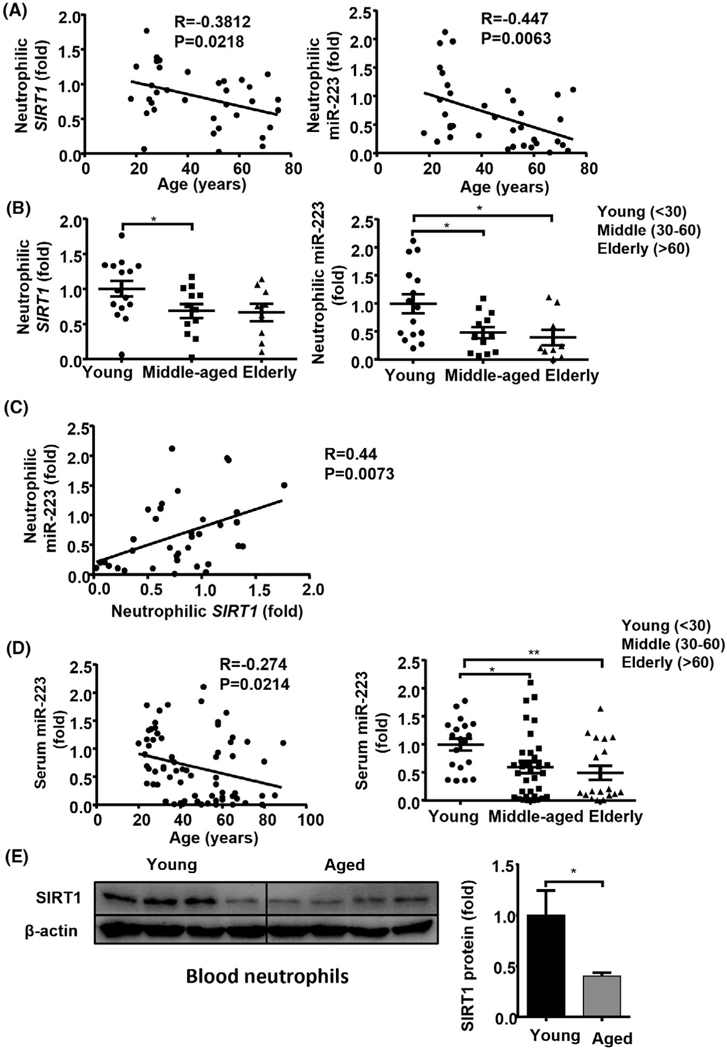
To determine if the down-regulation of neutrophilic SIRT1 and miR-223 during aging also occurs in humans, we analyzed neutrophilic SIRT1 and miR-223 in 36 healthy controls that were stratified into 3 age groups (young <30, middle-aged 30–60, and elderly >60)[40] (Table S1). Age had a positive correlation with baseline serum ALT, AST, and circulating neutrophils (Figure S6A). Within group comparison, the middle-aged subjects had higher levels of baseline ALT, AST, circulating neutrophils than the young group, the elderly subjects also had higher serum ALT levels and had trend higher levels of AST, circulating neutrophils but it did not reach statistical significance (Figure S6B). Furthermore, neutrophilic SIRT1 was negatively correlated with the percentage of circulating neutrophils, whereas neutrophilic miR-223 was negatively correlated with the total number of circulating neutrophils (Figure S6C). Interestingly, age had an inverse relationship with neutrophilic SIRT1 (r = −0.38, p = 0.02) or miR-223 (r = −0.45, p = 0.006) (as a continuous variable, Figure 7A). When compared between age groups, the middle-aged and/or elderly subjects had decreased neutrophilic expression of SIRT1 and miR-223 compared with the young group (Figure 7B). Remarkably, a positive correlation was observed between neutrophilic SIRT1 and miR-223 (r = 0.44, p = 0.007, Figure 7C). In addition, an inverse relationship between serum miR-223 (r = −0.27, p = 0.02) and age was observed (as a continuous variable, Figure 7D) in another cohort of 146 healthy controls (Table S2). Again, the middle-aged and elderly subjects had decreased serum miR-223 compared with the young group (Figure 7D). Finally, the SIRT1 protein levels were reduced in blood neutrophils from the elderly group compared with those from the young group (Figure 7E).
FIGURE 7.
Down-regulation of neutrophilic SIRT1 and miR-223, and serum miR-223 in middle-aged and elderly subjects. (A–C) Peripheral blood neutrophils were collected from young (n = 15), middle-aged (n = 12), and elderly (n = 9) healthy controls (Table S1) and were subjected to RT-qPCR analyses of SIRT1 and miR-223. Correlation analyses between age and neutrophilic SIRT1 or miR-223 (A); expression levels of SIRT1 and miR-223 (B); correlation analyses between neutrophilic SIRT1 and miR-223 (C). (D) Serum was collected from young (n = 67), middle-aged (n = 48) and elderly (n = 31) healthy controls (Table S2). Correlation analyses between age and serum miR-223 (left panel), RT-qPCR analyses of serum miR-223 (right panel). (E) Western blot analysis of SIRT1 protein levels from blood neutrophils in young (n = 4) and elderly (n = 4) healthy controls. Values represent means ± SEM. *p < 0.05; **p < 0.01
Acute-on-chronic drinking induces liver injury and regulates the SIRT1-miR-223 axis in neutrophils in young and middle-aged individuals
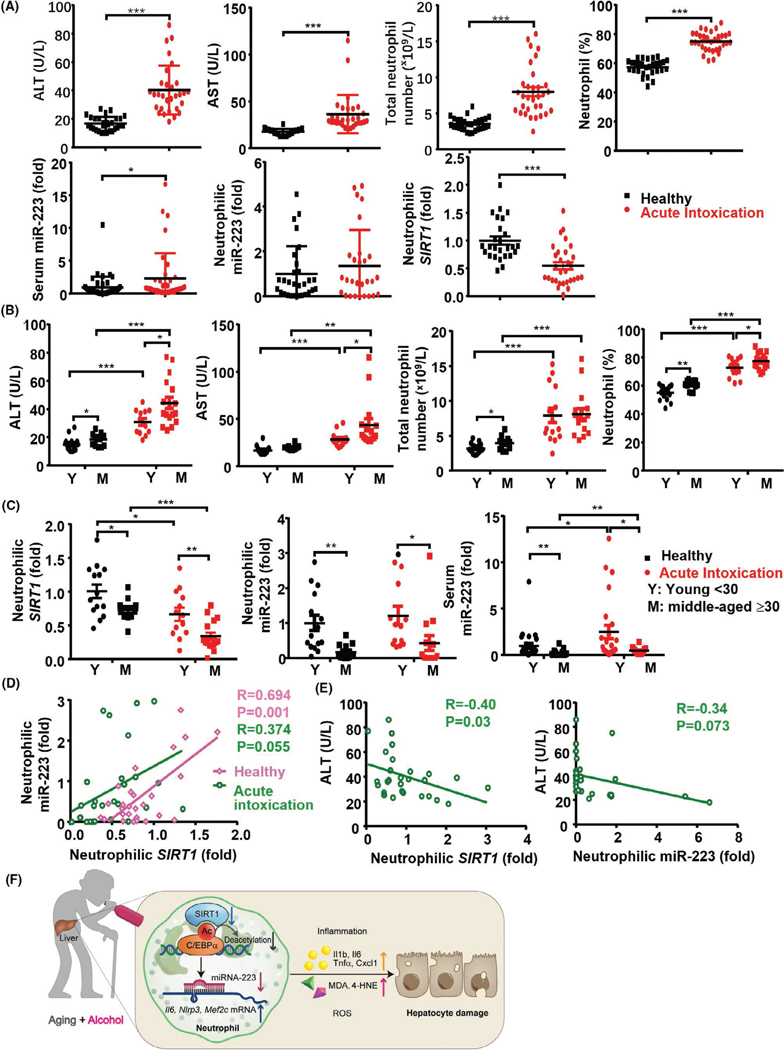
We analyzed the samples of healthy controls (N = 29) and patients with alcoholism (N = 32) who had a history of chronic alcohol drinking and presented to the Emergency Department with acute intoxication (Table S3). In intoxicated group, there was a trend of positive correlation between age and serum AST (r = 0.34, p = 0.069) but not serum ALT (r = 0.26, p = 0.15). However, in healthy controls, age was positively correlated with serum ALT and AST (Figure S7A). In addition, age had a strong positive correlation with the number and percentage of circulating neutrophils but had negative correlation with neutrophilic SIRT1 and miR-223, and serum miR-223 in both healthy and intoxicated groups (Figure S7B). Within group comparison, patients who were intoxicated had elevated levels of serum ALT, AST, circulating neutrophils, and serum miR-223, but a reduction of neutrophilic SIRT1 compared with healthy controls (Figure 8A), which are in agreement with our results from chronic-plus-binge ethanol feeding mouse model. To account for the effect of age, we further dichotomized patients with alcohol use disorder into young (<30) and middle-aged (≥30) groups (we did not have enough elderly patients who were intoxicated during this study) (Table S3). Within group comparison, serum ALT and AST levels, and the percentage of circulating neutrophils were higher in middle-aged than those in the young group with acute intoxication (Figure 8B). The percentage of circulating monocytes was inversely decreased in patients, but there was no reduction in the total number of circulating monocytes and lymphocytes, likely due to an increase in total white blood cells in these patients (Figure S8A).
FIGURE 8.
Acute-on-chronic drinking induces liver injury and regulates the SIRT1-miR-223 axis in neutrophils in young and middle-aged individuals. Blood samples from 29 healthy controls (16 young and 13 middle-aged people) and 32 patients with alcohol use disorder and acute intoxication (15 young and 17 middle-aged people) were analyzed (Table S3). (A) Serum ALT and AST levels, the total number and percentage of peripheral neutrophils, RT-qPCR analyses of neutrophilic SIRT1 and miR-223 and serum miR-2233 levels from healthy controls and patients with alcohol use disorder were measured. (B, C) The values in panel A were compared between different age groups (D) Correlation analyses between neutrophilic SIRT1 and miR-223 from healthy and intoxicated groups. (E) Correlation analyses between serum ALT levels and neutrophilic SIRT1 or miRNA-223 levels. Values in panels A–C represent means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. (F) A model depicting aging exacerbates ALD by inhibiting the neutrophilic SIRT1-miR-223 axis through the promotion of C/EBPα acetylation. ALD is associated with neutrophil infiltration, which promotes liver injury and inflammation by releasing ROS and inflammatory mediators. Interestingly, neutrophil-specific miR-223 plays a compensatory role in inhibiting ROS and inflammation. During aging, neutrophilic SIRT1 is down-regulated, such down-regulation results in decreased miR-223 expression through the induction of C/EBPα acetylation, and subsequent elevation of the miR-223 target genes including inflammatory genes (Il-6, Nlrp3, Mef2c), thereby exacerbating ALD
Next, we examined the neutrophilic SIRT1 and miR-223 levels and found patients with alcohol use disorder had a marked reduction in the expression of neutrophilic SIRT1 mRNA in both young and middle-aged groups compared with healthy controls, with much lower levels in the latter group (Figure 8C). Interestingly, neutrophilic miR-223 levels were lower in the middle-aged group than in the young group in both healthy and intoxicated groups, whereas there were no differences between healthy and intoxicated groups (Figure 8C). Serum miR-223 levels were higher in patients who were intoxicated than in healthy controls in both young and middle-aged subjects with much lower levels in the latter group (Figure 8C). Neutrophilic SIRT1 levels positively correlated with neutrophilic miR-223 levels in healthy controls (r = 0.69, p = 0.001), there was a trend positive correlation between neutrophilic SIRT1 and miR-223 in patients with alcohol use disorder (r = 0.37, p = 0.055) (Figure 8D). An inverse association was also observed between serum ALT and neutrophilic SIRT1 mRNA expression (r = −0.40, p = 0.03) but not with neutrophilic miR-223 (r = −0.34, p = 0.07) (Figure 8E). Moreover, in neutrophils from elderly patients with alcohol use disorder with acute intoxication, down-regulation of neutrophilic SIRT1 mRNA was associated with elevated acetylation of C/EBPα protein (Figure S8B), which is likely responsible for the decreased neutrophilic miR-223 in these subjects.
Finally, we examined other neutrophils-enriched miRNAs in addition to miR-223 in young and aged subjects, and intoxicated subjects. Consistent with the results from mice, there were no differences in miR-16, miR-143 a, miR-15a, and miR-27a expression in neutrophils from young and middle-aged subjects (Figure S9A, Table S4). There was also no correlation between these miRNA levels and ALT, AST, and neutrophil numbers except a negative correlation between neutrophilic miR-16a and the total number of circulating neutrophils, and a negative correlation between neutrophilic miR-15a and serum ALT levels or the number of circulating neutrophils (Figure S9B). Furthermore, there were no differences in miR-16, miR-142 a, miR-15a, and miR-27a expression in neutrophils between patients who were intoxicated and healthy controls (Figure S10A) (Table S4). An inverse correlation was observed between the percentage of circulating neutrophils and neutrophilic miR-142a or miR-27a expression but a positive correlation between the percentage of circulating neutrophils and neutrophilic miR-16a (Figure S10B).
DISCUSSION
Aging is a risk factor for various types of chronic liver diseases including ALD,[6–8] but the underlying mechanisms are poorly understood. In the current study, we have demonstrated neutrophilic SIRT1-miR-223 axis is down-regulated through C/EBPα acetylation in aged mice, and that similar down-regulation is also observed in middle-aged/elderly people. The reduction in SIRT1 and miR223 results in an increase in inflammatory mediators and ROS leading to liver injury in our mouse model and patients with chronic alcohol drinking presented with acute intoxication. The schematic diagram of the aging and neutrophilic SIRT1-miR-223 axis in ALD is shown in Figure 8F.
SIRT1 is a well-documented anti-aging molecule to control a wide variety of aging-related processes.[18–21] Aging is associated with the down-regulation of SIRT1, which in turn accelerates aging processes and disease progression.[18–21] We have previously demonstrated that SIRT1 is down-regulated in hepatocytes and HSCs in aged mice, and such down-regulation exacerbates ALD.[8] Down-regulation of the anti-aging molecule SIRT1 in the aged liver is consistent with more senescence phenotypes in the liver tissues from elderly than those from young people.[41] The present study has demonstrated that neutrophilic SIRT1 is similarly down-regulated in aged mice, and the deletion of the Sirt1 gene in neutrophils markedly enhances alcohol-induced liver injury and inflammation, suggesting that down-regulation of neutrophilic SIRT1 exacerbates ALD in aged mice. Down-regulation of SIRT1 in aged animals has been reported in various types of tissues including the liver, and several mechanisms have been implicated in SIRT1 down-regulation.[18–21] The specific mechanisms by which neutrophilic SIRT1 is down-regulated in aged mice were not examined in the current study, instead, we extensively investigated how SIRT1 regulates the downstream molecular pathway that affects neutrophil functions and ALD.
Given the important role of many miRNAs in regulating ALD pathogeneses,[34] we examined several neutrophil-specific miRNAs[35] and found that only miR-223 expression is markedly down-regulated in neutrophils isolated from Sirt1Mye−/− mice compared with those from WT mice, suggesting SIRT1 up-regulates miR-223 expression. Our studies supported that SIRT1 is an upstream regulator of miR-223 by promoting its biogenesis. First, a positive correlation was found between neutrophilic SIRT1 and miR-2 23 expression. Second, administration of the SIRT1 agonist RSV effectively increased miR-223 expression in neutrophils. Third, we demonstrated that SIRT1 can bind to C/EBPα and promote deacetylation of C/EBPα, a key transcription factor that promotes miR-2 23 expression,[30] thereby inducing miR-223 expression. Fourth, we found that the acetylation of C/EBPα was elevated in neutrophils from Sirt1Mye−/− mice compared with WT mice, providing in vivo evidence for a direct link between SIRT1, C/EBPα, and miR-223. Fifth, treatment with SIRT1 agonists inhibits C/EBPα acetylation, followed by an elevation of miR-223, whereas SIRT1 antagonists reduced miR-223 expression by promoting C/EBPα acetylation. Finally, C/EBPα acetylation is elevated in neutrophils from aged mice and ethanol-fed mice, which is likely responsible for the down-regulation of neutrophilic miR-223 in these mice. Interestingly, neutrophilic and serum miR-223 levels are elevated in young mice after ethanol feeding; the findings are likely due to an increase in inflammatory responses leading to an up-regulation of miR-223 in neutrophils. However, such miR-223 up-regulation is blunted in aged mice as indicated by comparable serum and neutrophilic miR-223 levels between ethanol-and pair-fed aged mice, which contributes to enhanced ALD in aged mice. In contrast to the down-regulation of neutrophilic miR-223, other neutrophils-enriched miRNAs including miR-27a, miR-15a, miR-142 and miR-143, have hardly changed in blood neutrophils from aged mice. Several studies have shown that miR-142 and miR-143 act as essential modulators of neutrophil development,[42,43] whereas the roles of miR-27a and miR-15a in neutrophils remain unknown. Therefore, dysregulation of these neutrophilic enriched miRNAs may also contribute to the enhanced susceptibility of aged mice to ALD. In addition, miR-34a has been shown to play a role in the pathogenesis of ALD in mice through the regulation of SIRT1 in the liver.[44] However, the role of neutrophilic miR-34a-SIRT1 axis in ALD pathogenesis has not been explored, which should be explored in the future study.
The current study also has clinical applicability. Our data revealed that aging in humans affects neutrophilic SIRT1 expression; its expression was markedly decreased in middle-aged and elderly people compared with those in young individuals. Neutrophilic and serum miR-223 levels were lower in middle-aged and elderly subjects than those in young people. Of importance, neutrophilic SIRT1, miR-223, and serum miR-223 levels are negatively correlated with age. Finally, SIRT1 levels were positively correlated with miR-223 levels in neutrophils. Collectively, our data strongly suggest that aging inhibits the SIRT1-miR-223 in neutrophils in humans similar to the results we observed in mice. In addition, we analyzed the blood test results and neutrophils in young and middle-aged individuals with chronic alcohol drinking presented with acute intoxication, which mimics chronic-plus-binge ethanol feeding in mice. Interestingly, these patients had many similar features that we observed in the chronic-plus-binge ethanol feeding model, such as elevation of serum ALT and AST, and circulating neutrophils in both young and middle-aged groups after acute intoxication with greater elevation in the latter group, suggesting that middle-aged people are more susceptible to alcohol-induced liver injury than young people. One of the reasons for the increased susceptibility to ALD in middle-aged people is probably due to the decreased neutrophilic SIRT1-miR-223 as demonstrated in the current study. Given an important anti-inflammatory and antifibrotic function of miR-223,[14–16] down-regulation of neutrophilic miR-223 likely contributes to the elevated circulating neutrophils and increased liver injury in middle-aged people than those in young people. In addition, our data from the study of mouse models indicate that miR-223 expression in neutrophils is controlled by SIRT1, which is probably also true in humans because neutrophilic miR-223 levels positively correlate with neutrophilic SIRT1 in both young and middle-aged people without alcohol drinking, there is also a trend in positive correlation (p = 0.055) between these two groups after acute alcohol intoxication. In summary, neutrophilic SIRT1 is also down-regulated during aging in humans and such down-regulation likely contributes to the increased susceptibility of ALD, therefore, neutrophilic SIRT1 could be a therapeutic target for the prevention and/or treatment of this disease.
Supplementary Material
Acknowledgments
Funding information
This study was supported in part by the National Nature and Science Foundation (81770588 to HW) and by the intramural program of the NIAAA, NIH, USA (BG). Dr. Hua Wang and Dr. Bin Gao are co-mentors for Ms. Ruixue Ren, who was a participant in the NIH Graduate Partnerships Program and a graduate student in the Anhui Medical University when she worked on this project during 2018-2020 and was affiliated with the Anhui Medical University and the NIH Graduate Partnerships Program (GPP)
Abbreviations:
- 4-HNE
4-hydroxynonenal
- ALD
alcohol-associated liver disease
- ALT
alanine aminotransferase
- BM
bone marrow
- C/EBPα
CCAAT/enhancer binding protein α
- MPO
myeloperoxidase
- NCF
neutrophil cytosolic factor
- NLRP3
NOD-, LRR-, and pyrin domain-containing protein 3
- pri-miR-223
primary miR-223
- ROS
reactive oxygen species
- RSV
resveratrol
- SIRT1
sirtuin 1
- Sirt1f/f
littermate control
- Sirt1Mye−/−
myeloid-cell specific Sirt1 knockout
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
CONFLICT OF INTEREST
Nothing to report.
REFERENCES
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. [DOI] [PubMed] [Google Scholar]
- 2.Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71(1):212–21. [DOI] [PubMed] [Google Scholar]
- 3.Avila MA, Dufour JF, Gerbes AL, Zoulim F, Bataller R, Burra P, et al. Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut. 2020;69(4):764–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306–33. [DOI] [PubMed] [Google Scholar]
- 5.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheedfar F, Biase SD, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12(6):950–4. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez T, Li YM, Yin S, Xu M-J, Feng D, Zhou Z, et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66(3):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol. 2014;29:23–8. [DOI] [PubMed] [Google Scholar]
- 10.Adrover JM, Nicolás-Ávila JA, Hidalgo A. Aging: a temporal dimension for neutrophils. Trends Immunol. 2016;37(5):334–45. [DOI] [PubMed] [Google Scholar]
- 11.Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58(5): 1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho Y, Szabo G. Two faces of neutrophils in liver disease development and progression. Hepatology. 2021;74(1):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Rodrigues RM, Wang X, Seo W, Ma J, Hwang S, et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J Clin Invest. 2021;131(3):e141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvente CJ, Tameda M, Johnson CD, del Pilar H, Lin YC, Adronikou N, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest. 2019;129(10):4091–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Hwang S, Cai Y, Kim S-J, Xu M, Yang D, et al. MicroRNA-223 ameliorates nonalcoholic steatohepatitis and cancer by targeting multiple inflammatory and oncogenic genes in hepatocytes. Hepatology. 2019;70(4):1150–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boule LA, Kovacs EJ. Alcohol, aging, and innate immunity. J Leukoc Biol. 2017;102(1):41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84(10):1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev. 2015;146–148:28–41. [DOI] [PubMed] [Google Scholar]
- 20.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Lee JH, Lee HY, Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol Res. 2013;67(1):60–7. [DOI] [PubMed] [Google Scholar]
- 23.Hwang J-W, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30(19):4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade JMO, Paraíso AF, de Oliveira MVM, Martins AME, Neto JF, Guimarães ALS, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30(7–8):915–9. [DOI] [PubMed] [Google Scholar]
- 26.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H, Hu M, Liang X, Ajmo JM, Li X, Bataller R, et al. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 2014;146(3):801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Ye TJ, DeCaro E, Buehler B, Stahl Z, Bonavita G, et al. Intestinal SIRT1 deficiency protects mice from ethanol-induced liver injury by mitigating ferroptosis. Am J Pathol. 2020;190(1):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren R, Wang Z, Wu M, Wang H. Emerging roles of SIRT1 in alcoholic liver disease. Int J Biol Sci. 2020;16(16):3174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukao T, Fukuda Y, Kiga K, Sharif J, Hino K, Enomoto Y, et al. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell. 2007;129(3):617–31. [DOI] [PubMed] [Google Scholar]
- 31.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8(3):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124(3):778–90. [DOI] [PubMed] [Google Scholar]
- 33.Dimmeler S, Nicotera P. MicroRNAs in age-related diseases. EMBO Mol Med. 2013;5(2):180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70(4):784–95. [DOI] [PubMed] [Google Scholar]
- 35.Gantier MP. The not-so-neutral role of microRNAs in neutrophil biology. J Leukoc Biol. 2013;94(4):575–83. [DOI] [PubMed] [Google Scholar]
- 36.Li M, He Y, Zhou Z, Ramirez T, Yu Gao, Ya Gao, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut. 2017;66:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. [DOI] [PubMed] [Google Scholar]
- 38.Zaini MA, Muller C, de Jong TV, Ackermann T, Hartleben G, Kortman G, et al. A p300 and SIRT1 regulated acetylation switch of C/EBPalpha controls mitochondrial function. Cell Rep. 2018;22:497–511. [DOI] [PubMed] [Google Scholar]
- 39.Li YU, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146(2):539–49.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu R, Gong CX, Duan CM, Huang JC, Yang GQ, Yuan JJ, et al. Age-dependent changes in the plasma proteome of healthy adults. J Nutr Health Aging. 2020;24(8):846–56. [DOI] [PubMed] [Google Scholar]
- 41.Hunt NJ, Kang SW, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of aging in the liver. Comput Struct Biotechnol J. 2019;17:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan HB, Liu YJ, Wang L, Du TT, Dong M, Gao LI, et al. miR-142–3 p acts as an essential modulator of neutrophil development in zebrafish. Blood. 2014;124(8):1320–30. [DOI] [PubMed] [Google Scholar]
- 43.Batliner J, Buehrer E, Fey MF, Tschan MP. Inhibition of the miR-143/145 cluster attenuated neutrophil differentiation of APL cells. Leuk Res. 2012;36(2):237–40. [DOI] [PubMed] [Google Scholar]
- 44.McDaniel K, Herrera L, Zhou T, Francis H, Han Y, Levine P, et al. The functional role of microRNAs in alcoholic liver injury. J Cell Mol Med. 2014;18:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.