Abstract
A stressor can trigger lasting adaptations that contribute to neuropsychiatric disorders. Predator odor (TMT) exposure is an innate stressor that may activate the metabotropic glutamate receptor 3 (mGlu3) to produce stress adaptations. To evaluate functional involvement, the mGlu3 negative allosteric modulator (NAM, VU6010572; 3 mg/kg, i.p.) was administered before TMT exposure in male, Long Evans rats. Two weeks after, rats underwent context re-exposure, elevated zero maze (ZM), and acoustic startle (ASR) behavioral tests, followed by RT-PCR gene expression in the insular cortex and BNST to evaluate lasting behavioral and molecular adaptations from stressor. Rats displayed stress-reactive behaviors in response to TMT exposure that were not affected by VU6010572. Freezing and hyperactivity were observed during the context re-exposure, and mGlu3-NAM pretreatment during stressor prevented the context freezing response. TMT exposure did not affect ZM or ASR measures, but VU6010572 increased time spent in the open arms and ASR habituation regardless of stressor treatment. In the insular cortex, TMT exposure increased expression of mGlu (Grm3, Grm5) and NMDA (GriN2A, GriN2B, GriN2C, GriN3A, GriN3B) receptor transcripts, and mGlu3-NAM pretreatment blocked GriN3B upregulation. In the BNST, TMT exposure increased expression of GriN2B and GriN3B in vehicle-treated rats, but decreased expression in the mGlu3-NAM group. Similar to the insular cortex, mGlu3-NAM reversed the stressor-induced upregulation of GriN3B in the BNST. mGlu3-NAM also upregulated GriN2A, GriN2B, GriN3B and Grm2 in the control group, but not the TMT group. Together, these data implicate mGlu3 receptor signaling in some lasting adaptations of predator odor stressor and anxiolytic-like effects.
Keywords: stress, glutamate, NR3B, VU6010572, Insular Cortex, BNST
1. Introduction
Stress-related neuropsychiatric disorders are associated with dysregulated glutamatergic function [1–6]. Stress directly alters glutamatergic neurotransmission through glucocorticoid-receptor (GR) binding on glutamate neurons [7]. For example, stress models (e.g., forced-swim, tail-suspension, foot shock, glucocorticoid treatment) have repeatedly been shown to acutely increase presynaptic glutamate release in the brain [7–14]. Further, acute stressor exposure (e.g., forced-swim, tail suspension, elevated-plus maze) potentiates N-methyl-D-aspartate receptor (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor currents and surface expression through a GR-dependent mechanism on medial prefrontal cortex (mPFC) pyramidal neurons [15]. Overall, stress has been shown to have a multitude of acute and lasting effects specifically on cortico-limbic glutamate function that potentially contribute to maladaptive responses that increase vulnerability for the development of neuropsychiatric disorders [7, 16, 17].
Metabotropic glutamate (mGlu) receptors are potential therapeutic targets for many neuropsychiatric disorders [1–3, 6, 18], and modulate excitatory cell function through intracellular cascade events that can result in lasting adaptations to the cell in response to stressors [6, 19]. Group II mGlu receptors (mGlu2 and mGlu3) are coupled to Gi-proteins that reduce cellular activity as a result of activation [6]. Traditionally, Group II mGlu receptors are considered predominantly located on presynaptic terminals and their activation attenuates presynaptic neurotransmitter release [6]. However, mGlu3 receptors have been found on both pre- and post-synaptic neurons, as well as astrocyte projections in the brain [20, 21]. Regardless of localization, mGlu2/3 receptor blockade has consistently been shown to have antidepressant- and antianxiety-like behavioral effects in rodents induced by stress with overlapping brain mechanisms to that of ketamine and other glutamate-targeting therapeutics [16, 17, 22–27]. Furthermore, the glutamate system has been shown to play an important role in anti-depressant drug effects [12, 28–30]. Therefore, mGlu2/3 receptor blockade has been proposed as an alternative to ketamine for its therapeutic potential without the psychogenetic and addiction-liability risks associated with ketamine [17]. The mechanisms by which mGlu2 and mGlu3 signaling exert their therapeutic effects are important for understanding novel mechanisms of glutamate-targeting antidepressant-like effects. However, prior to the recent development of highly specific negative allosteric modulators (NAMs) for mGlu3 receptors [31, 32], the specific role of mGlu3 vs. mGlu2 receptors in these effects had been elusive. The same drug class of mGlu3 NAMs that were used in these experiments (VU6010572, first generation: VU0650786) have recently been employed to show that mGlu3 mediates a form of synaptic plasticity in cortico-limbic circuitry that is sensitive to stress [19, 20, 27, 31] and exhibits rapid anti-depressant-like effects [27]. Restraint stress before electrophysiological measurements occluded mGlu3-mediated long-term depression (LTD) in a manner consistent with mGlu3 loss of function. Prior studies show that blocking mGlu3 activation in vivo prior to restraint stress restored mGlu3-LTD [19]. As previously mentioned, mGlu2/3 receptor blockade reverses stress-induced depressive-like phenotypes in rodents [17]. Therefore, the hypothesis tested here is that synaptic plasticity induced by mGlu3-signaling during a stress event plays a role in the lasting behavioral and glutamatergic adaptations of stressor exposure.
Cortico-limbic circuitry is implicated in stress-related neuropsychiatric disorders. In animal models, the insular cortex and the bed nucleus of the stria terminalis (BNST) both play an important role in stress responses [33, 34]. The insular cortex is commonly associated with the integration of sensory and interoceptive processing [35, 36], which is highly sensitive to stress and anxiety in preclinical models [37, 38] and implicated in the etiology of neuropsychiatric disorders [39]. For example, inactivation of the anterior insular cortex produced anxiogenic-like effects, whereas activation of the insular cortex produced anxiolytic-like effects as assessed by the elevated plus maze test [37]. Clinically, anticipation of unpredictable stress increased BOLD signal in the insular cortex as assessed by fMRI [34]. The BNST is also associated with stress-related neuropsychiatric disorders [40] and animals models of stress adaptations [41]. For example, restraint stress in mice increased c-Fos immunoreactivity in CRF-expressing neurons of the dorsal BNST [42]. Additionally, inactivation of the BNST during predator odor exposure stress blocked stressor-induced freezing behavior in rodents [43], indicating involvement in the fear-like response to TMT. Finally, the insular cortex and BNST show reciprocal projections that are likely involved in the lasting plasticity from a stress response [44]. Therefore, the focus of the present work is on glutamatergic gene expression adaptations following stressor exposure in the insular cortex and BNST. Genes that encode for glutamate receptors were evaluated: NMDA: GriN1, GriN2A, GriN2B, GriN2C, GriN2D, GriN3A, GriN3B, and mGlu: Grm2, Grm3, and Grm5.
Animal models have used exposure to the scent of a predator as a stressor that produces lasting behavioral consequences with relevance to stress-related neuropsychiatric disorders [45–58]. For example, exposure to the synthetically derived fox odor 2,5- dihydro- 2,4,5-trimethylthiazoline (TMT) produces avoidance and freezing behaviors, indicative of a fear- like response and increased serum corticosterone, reflecting a stress response [50, 53, 59, 60]. Other studies have showed lasting anxiety- like and hyperarousal behavior, as well as avoidance of a TMT- paired context or cue [46, 54]. Predator odor animal models also capture the acute and long-term effects of glutamatergic adaptation to stress. For example, a ferret predator odor was shown to increase glutamate efflux in the CeA of rats [61]. Furthermore, predator odor adaptations to NMDA receptors in CeA circuitry mediate some of the lasting behavioral effects of the stressor [62]. Our lab recently showed that TMT exposure also produces lasting adaptations (4 weeks after TMT) in glutamate-related gene expression (Grm2, Grm5, Grm7, Shank3, Homer1, Slc1A3) in corticolimbic brain regions [63]. Of relevance to the present work, we have reported decreased Grm3 gene expression 2 days after TMT exposure [63]. Given recent physiology findings showing that mGlu3 activation results in subsequent loss of function [29], we hypothesized that the decrease in Grm3 following TMT exposure may reflect a transcriptional mechanism for loss of function following stressor-induced activation of mGlu3. Furthermore, mGlu3 receptor activation during stressor may be involved in lasting adaptations to glutamatergic cortico-limbic circuitry [16, 17, 23–26]. Therefore, the present study blocks mGlu3 signaling during the TMT exposure to evaluate the functional involvement of mGlu3 activation during a stressor.
In this paper we used the newly developed mGlu3 NAM VU6010572 to block mGlu3 receptor signaling. Prior reports demonstrate that VU6010572 shows high specificity for mGlu3 receptors and high CNS permeability (brain/plasma Kp = 1.2), overcoming a common problem in the investigation of the specific involvement of mGlu3 receptor signaling from that of mGlu2 receptor signaling [32]. High selectivity for mGlu3 receptors has been shown (mGlu3 IC50 = 245 nM; mGlu2 IC50 > 30uM; mGlu5 IC50 > 30 uM), which also did not have off-target effects in “a Eurofins radioligand binding panel of 68 GPCRs, ion channels, transporters, and nuclear hormone receptors.” [32] Additionally, studies show that this drug class blocks LTD induced by mGlu2/3 agonists [19, 20] and produces acute antidepressant like effects (tail suspension test) in mice at a dose of 3 mg/kg [32]. Therefore, this was the justification for choosing the 3 mg/kg dose in these studies. Together, these data demonstrate high confidence that VU6010572 can be used as a tool to reduce mGlu3 signaling in the brain.
The goal of the present work was to block mGlu3 receptor activation (using VU6010572) during the TMT stressor and evaluate its functional implications on the lasting adaptations as a consequence of stressor. To capture the lasting behavioral and molecular consequences of the TMT stressor, rats remained undisturbed in the home cage for 2 weeks (operationally defined here as an “incubation period”) before behavioral testing (context re-exposure, zero maze, and acoustic startle response) and molecular assessments (gene expression for glutamate receptor transcripts) were conducted. Therefore, these experiments provide insight into the role of mGlu3-signaling during a stressor on lasting stressor-induced adaptations, and in doing so also evaluate the potential long-term therapeutic effects of the mGlu3-NAM VU6010572. These data could provide us with greater mechanistic insight into how stressor may influence vulnerability to the development of a neuropsychiatric disorder and the adaptations that result from mGlu3 signaling during stressor exposure.
2. Methods
2.1. Animals
Male Long- Evans rats (n = 48; Envigo, Indianapolis, IN) were used for all experiments. Rats arrived at 7 weeks and were handled for at least 1 minute daily for 1 week prior to experiments. To obviate potential stress-transfer effects, all rats arrived and remained single-housed throughout the duration of experiments, consistent with our previous publications [63]. Rats were housed in ventilated cages with access to food and water ad libitum and maintained in a temperature and humidity-controlled vivarium with a 12-hour light/dark cycle. All experiments were conducted during the light cycle. Rats were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine at UNC- Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
2.2. Compounds
VU6010572 was provided by Dr. Craig Lindsley and synthesized according to [32]. VU6010572 was dissolved in 45% β-cyclodextrin. A 1.5 mg/mL solution was made and injected at a volume of 2 mL/kg intraperitoneally (i.p.) to achieve a 3 mg/kg dose. An equal volume of 45% β-cyclodextrin was used as vehicle. The predator odor used was 2,5- dihydro- 2,4,5-trimethylthiazoline (TMT; 97% purity; SRQ Bio, Sarasota, FL).
2.3. Assessment of mGlu3-NAM pretreatment on TMT exposure stress reactivity
Rats (n=12/grp) were injected with VU6010572 (0 or 3 mg/kg, i.p.) and returned to the home cage for 45 mins prior to TMT-exposure testing. TMT exposure test chambers and experimental set-up were identical to those used in [59, 63]. Briefly, rats were transported from the vivarium in the home cage to a separate, well- ventilated room that contained the test chambers in which rats were exposed to TMT (45.72 × 17.78 × 21.59 cm; UNC Instrument Shop, Chapel Hill, NC). Only one rat was placed in each chamber. The length of the back wall of the test chambers was opaque white with two opaque black side walls and a clear, plexiglass front wall to enable video recordings and a clear sliding lid. The bottom of the chamber was covered with a layer of white bedding [63]. A small, metal basket was hung on the right- side wall (17.8 cm above the floor) to hold a piece of filter paper. 10 μL of TMT (2,5- dihydro- 2,4,5- trimethylthiazoline) or water for controls was pipetted onto the filter paper in the metal basket immediately prior to putting the rat in the chamber. The control group was always run before the TMT group to prevent odor contamination. The odor exposure session lasted 15 mins and was video recorded for evaluation of behavior using ANY- maze Video Tracking System (Version 6.12, Stoelting Co. Wood Dale, IL). After TMT exposure, rats were returned to the home cage and remained undisturbed in the home cage for 2 weeks as an incubation period prior to starting behavioral assessments. Two rats that were initially assigned to the TMT/mGlu3-NAM group were moved to the TMT/vehicle group prior to injection due to unintentional loss of mGlu3-NAM solution. One other animal in the TMT/mGlu3-NAM group was removed from the study due to a failed i.p. injection. Final sample sizes: (n=12 - ctrl/vehicle; n=12 - ctrl/mGlu3-NAM; n=14 - TMT/vehicle; n=9 - TMT/mGlu3-NAM).
2.4. Context Re-exposure
Two weeks (14 days) following TMT exposure, rats were returned to the same test chambers in which they had been previously exposed to TMT. No TMT was used for this context re-exposure test. Identical to the TMT exposure, this test lasted 15 min in duration and was video recorded for behavioral assessments identical to the TMT exposure behavioral measures.
2.5. Elevated Zero Maze
24 hours after the context re-exposure test (15 days post-TMT exposure), rats were evaluated on an elevated zero maze. Rats were transported in their home cages to the behavioral testing room and allowed to acclimate for 30 min prior to testing. The zero maze was made up of a circular platform with a diameter of 99 cm raised above the floor to a height of approximately 70 cm. The maze was divided equally into four quadrants/arms. Two enclosed arms contained two walls with one front wall [33.02 cm (H)] and back wall [64.52 cm (H)]. The exposed arms were located on opposite sides of the circular platform and were bordered with approximately 5 cm rim to prevent the rat from falling off the circular platform. Rats were placed in one open arm at the start of the 5 min test. These experiments were video recorded for analysis.
2.6. Acoustic Startle Response
24-hours after the zero-maze test (16 days pots-TMT exposure), rats were evaluated on an acoustic startle response test using an acoustic startle response system (S-R Lab; San Diego Instruments, San Diego, CA). Rats were transported in their home cages to the behavioral testing room and allowed to acclimate for 30 min prior to testing. Rats were placed in a cylinder Plexiglas animal enclosure located within a sound-attenuating test chamber that included an exhaust fan, a sound source, and an internal light that was turned off during the test. At the start of each test, rats underwent a 5-min habituation period during which 60 dB of background white noise was present. The background noise was present during the entire test session. The test session consisted of 30 trials of a 100 ms burst of a 110 dB startle. Each trial was separated by a 30 to 45-s randomized intertrial interval. Startle response (amplitude) was measured with a high-accuracy accelerometer mounted under the animal enclosure and analyzed with SR-Lab software.
2.7. Brain tissue collection and sectioning
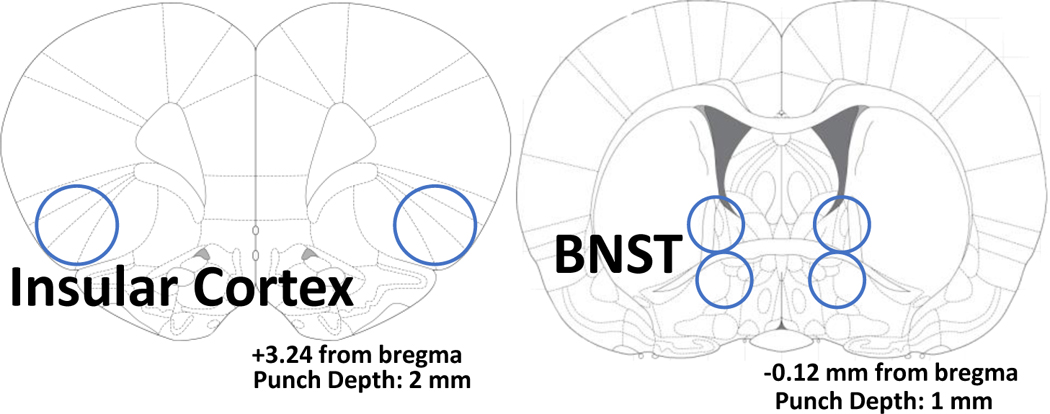
24 hours after the acoustic startle test (17 days post-TMT exposure), a sub-set of rats (n=8 control/vehicle, n=5 control/mGlu3-NAM, n=12 TMT/vehicle, n=9 TMT/mGlu3-NAM) were sacrificed for qPCR analysis. More rats were allocated to the TMT group because previous studies showed that TMT exposure can produce distinct sub-groups within the TMT-exposed group that differ in brain gene expression [54]. Rats were anesthetized in 5% isoflurane immediately prior to brain extraction. Brains were rapidly extracted, and flash frozen with isopentane (Sigma- Aldrich, MI), then stored at −80 °C until brain region sectioning. Brains were sectioned on a cryostat (−20 °C) up to a predetermined bregma for each region of interest (ROI) according to [64]. Then, a micropunch tool was used to remove tissue specific to each brain region as illustrated in Table 1. Brain sections were stored at −80°C until qPCR analysis.
Table 1 –
qPCR Tissue extraction and Primer Sequences
| ||
|---|---|---|
| Primer | Forward (5’−3’) | Reverse (5’−3’) |
| GriN1 | CTATGACAACAAGCGCGGAC | GCCCGTCATGTTCAGCATTG |
| GriN2A | GCGGGAACCCGCTAAACC | GCAATACCAGCAAGGTCCAGT |
| GriN2B | GGGTCACGCAAAACCCTTTC | CCTTGTTTTTGACGCCCCTG |
| GriN2C | CAACGTCTTGGTTCCCCTCA | CTTGGGCTTCTCCTCTCAGC |
| GriN2D | CCTCATGGGTTGGGAAGGAC | TCTGGCTCATAGTTCCCCCA |
| GriN3A | TCTCTCCATCCTGACCACCAT | CCATCTTCTCCATCTGCTCTTCT |
| GriN3B | ACCCTGTACGAATGGCGAAG | GTGCGTCCAAAGAGAATGGC |
| Grm2 | CCTACAATGTGCTCCTCATCGC | CTGACCTAAAGGGCTGGGAATC |
| Grm3 | ATTCTCAGTCCTCTGCAAGC | AGACCCTGTCACCAATGCTC |
| Grm5 | CTGGCCTTCGTGCCTATCTA | TTTCCGTTGGAGCTTAGGGTT |
| β-Actin | CTACAATGAGCTGCGTGTGGC | CAGGTCCAGACGCAGGATGGC |
qPCR Analysis
2.8. RNA Extraction-
RNA was extracted from brain tissue using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. RLT lysis buffer containing β- mercaptoethanol (Sigma Aldrich) was used for tissue homogenization. RNA concentration and purity for each sample were determined using a Spectrophotometer (Nanodrop 2000, ThermoScientific).
2.9. Reverse Transcription-
RNA was reverse transcribed into cDNA using the SuperScript™ III First-Strand Synthesis System (ThermoFisher Scientific) according to the manufacturer’s instructions. Following reverse transcription, all samples were diluted 1:5 with water and stored at −20°C before RT- PCR experiments.
2.10. RT- PCR-
The QuantStudio3 PCR machine (ThermoFisher) was used for all experiments. Using a 96- well plate, each sample was run in triplicate using 10 μL total volume per well with the following components: PowerUp Syber green dye (ThermoFisher, containing ROX dye for passive reference), forward and reverse primers (Eton Biosciences Inc., NC) and cDNA template. The PCR was run with an initial activation for 10 minutes at 95 °C, followed by 40 cycles of the following: denaturation (95°C for 15 seconds), annealing/extension (60°C for 60 seconds). Melt curves were obtained for all experiments to verify synthesis of a single amplicon. All primers targeted genes that encode for glutamate receptors and include: GriN1, GriN2A, GriN2B, GriN2C, GriN2D, GriN3A, GriN3B, Grm2, Grm3, and Grm5. β-actin was used as the housekeeper gene. All primer sequences are displayed in Table 1.
3. Data Analysis
3.1. TMT Exposure and Context Re-exposure.
Analysis of TMT exposure and context re-exposure behaviors are identical to [63] except for freezing analysis included in the present work. Using ANY- maze, the length of the rectangular TMT exposure chamber was divided into two compartments for analysis (TMT side and non- TMT side). The basket containing TMT was located on the far end of the TMT side. Dependent measures assessed via ANY-maze included time spent of the TMT side of the chamber, time spent digging, time spent grooming, distance traveled and midline crossings (the number of times the animal crossed between the TMT and non-TMT side). Freezing was operationally defined as the absence of movement except respiration while the animal was not resting/sleeping, identical to [54]. Time spent digging in the bedding (see [59, 63]), time spent freezing, and time spent grooming were quantified manually by an experimenter blind to experimental conditions. For the context re-exposure, two rats were removed from analysis due to a camera recording error. Context re-exposure sample sizes: (n=11 - control/vehicle; n=12 - control/mGlu3-NAM; n=13 - TMT/vehicle; n=9 - TMT/mGlu3-NAM).
These dependent measures were analyzed by two- way ANOVAs and Sidak’s multiple comparison test was used for all post hoc analyses, other than for freezing analysis in the context re-exposure. Based on the a priori hypothesis and the literature that TMT exposure would increase freezing upon re-exposure to the context, planned comparisons were conducted to investigate the effects of TMT exposure in both the vehicle and mGlu3-NAM treated groups (student’s t-test). Pearson correlational analyses were conducted between stress-reactive behaviors to the TMT exposure and to the context re-exposure reactivity in the TMT/Vehicle group. Positive correlations identified in the TMT/Vehicle group were evaluated in all other experimental groups.
3.3. Zero Maze.
The time spent in the open arms, number of open and closed arm entries, total number of entries (open + closed), and total distance traveled were quantified using ANY-maze software and analyzed by two- way ANOVA with Sidak’s multiple comparison test for post hoc analyses. Three rats were excluded from this analysis – two due to falling off of the zero maze, and another was determined to be a statistically significant outlier. Sample sizes: (n=10 - control/vehicle; n=12 - control/mGlu3-NAM; n=13 - TMT/vehicle; n=9 - TMT/mGlu3-NAM).
3.4. Acoustic Startle Response.
The peak startle response (amplitude) was averaged for all 30-trials. In order to assess how startle response changes over time, and to evaluate habituation, we averaged the first and last 15-trials. Next, we calculated the percent change between the average of the first 15 trials and last 15 trials as follows:
This measure was defined as “Habituation.” Two rats were removed from this analysis due to an experimental error. Sample sizes: (n=11 - control/vehicle; n=11 - control/mGlu3-NAM; n=13 - TMT/vehicle; n=9 - TMT/mGlu3-NAM). Data were analyzed by two- way ANOVA with Sidak’s multiple comparison test for post hoc analyses.
3.5. Gene expression assessments.
The ΔΔCt method was used to determine fold change relative to controls using β-actin as the housekeeper gene [65]. Fold changes were normalized so that average control fold change equaled 1. Samples sizes for gene expression analysis started as: n=8 control/vehicle; n=5 control/mGlu3-NAM; n=12 TMT/vehicle; n=9 TMT/mGlu3-NAM (see methods). Two rats were removed from analysis due to brain extraction errors. Additionally, two of the control/vehicle samples ran out of cDNA before testing all genes, making the final samples sizes for the insular cortex: n=6–8 control/vehicle; n=5 control/mGlu3-NAM; n=12 TMT/vehicle; n=7 TMT/ mGlu3-NAM. For the BNST, 4 additional samples were lost as a result of RNA isolation errors, resulting in n=8 - control/vehicle; n=4 control/mGlu3-NAM; n=9 TMT/vehicle; n=7 TMT/mGlu3-NAM. Data were analyzed using 2-way ANOVAs, with Sidak’s multiple comparison test for post hoc analyses. All gene expression data are reported as mean fold change ± SEM. For all analyses, significance was set at P ≤ 0.05.
3.6. Power Analysis and Effect Sizes
Sample sizes (N=12/group; total 48) were determined using G-power analysis incorporating both predicted variability and effect size necessary to detect statistically significant differences based on our published work and other data from the lab. With a large effect size of f=0.45 (determined from prior effects of TMT exposure), α set at 0.05, a power of 0.80, and 4 groups, a total sample size of 41 rats was determined using G-plot. Therefore, these experiments started with 12 rats per group (total 48) to account for potential animal loss. All statistically significant results include effect sizes as eta squared (η2). η2 is used in multi-factor analyses as a metric of the proportion of the total variability that is attributable to the factor of interest [66]. Small, medium, and large sample size delineation is based off of Cohen’s d definitions of effect size magnitude that correspond with η2. Effects are defined as a small effect (η2=0.009), medium effect (η2=0.0588), and large effects (η2=0.1379) [66].
4. Results
4.1. Timeline of experiment
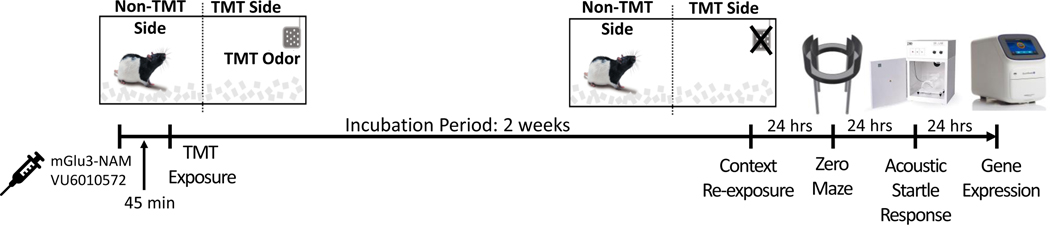
Figure 1 shows the experimental timeline.
Figure 1.
Experimental Timeline
4.2. TMT exposure produces stress-reactive behaviors that are not affected by mGlu3-NAM
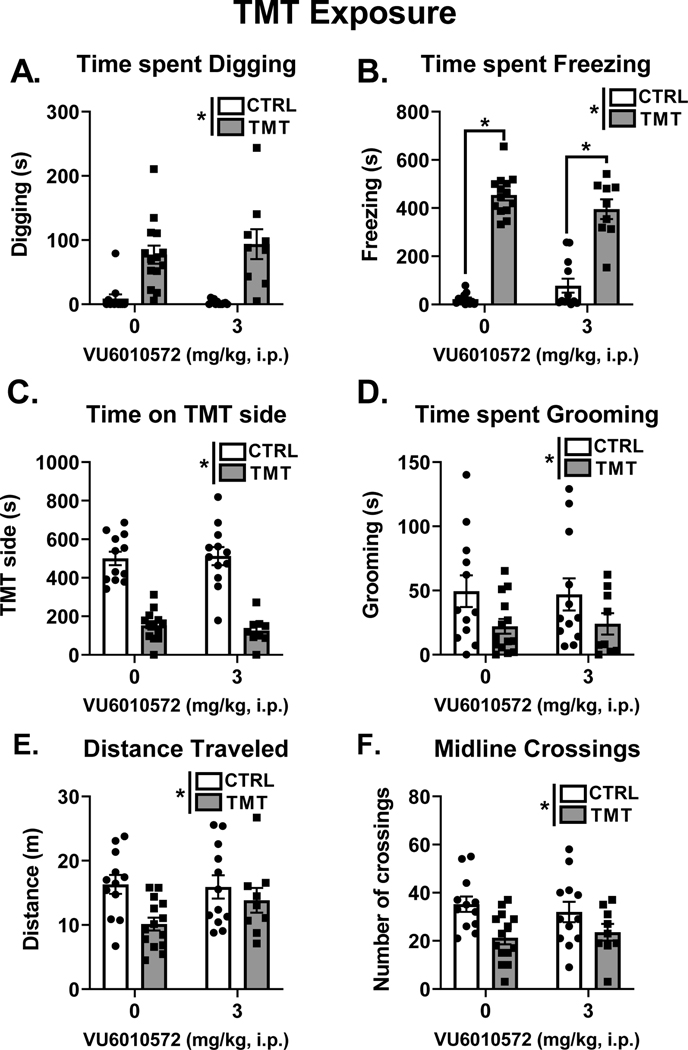
Figure 2 A–F shows the behavioral responses during TMT exposure. All behavioral measures (e.g., digging, freezing, time spent on the TMT side, grooming, distance traveled and midline crossings) showed a main effect of TMT exposure and no significant effect of mGlu3-NAM. TMT exposure increased time spent digging (Fig. 2A, F (1, 43) = 38.32, p < 0.0001, η2=0.46) and time spent freezing (Fig. 2B, F (1, 43) = 210.10, p < 0.0001, η2=0.77). For freezing, we observed a significant interaction effect between TMT exposure and mGlu3-NAM pretreatment (F (1, 43) = 4.92, p = 0.03, η2=0.02), with both the vehicle (p < 0.0001) and mGlu3-NAM (p < 0.0001) groups that were exposed to TMT showing increased freezing compared to respective control groups. TMT exposure decreased time spent on the TMT side of the test chamber (Fig. 2C, F (1, 43) = 61.55, p < 0.0001, η2=0.72) and time spent grooming (Fig. 2D, F (1, 43) = 6.02, p = 0.01, η2=0.12). Distance traveled (Fig. 2E, F (1, 43) = 7.15, p = 0.01, η2=0.13) and midline crossings (Fig. 2F, F (1, 43) = 10.44, p = 0.002, η2=0.19) were decreased as a main effect of TMT exposure. These results demonstrate robust behavioral stress-reactivity to TMT exposure that is not affected by pretreatment with the mGlu3-NAM.
Figure 2. TMT exposure produces stress-reactive behaviors that are not affected by mGlu3-NAM.
TMT exposure increased time spent digging (A) and time spent freezing (B). TMT exposure decreased time spent on the TMT side (C), time spent grooming (D), distance traveled (E) and the number of midline crossings (F). mGlu3-NAM did not affect any of these measures * p≤0.05 significantly different from Control.
4.3. TMT exposure produces freezing and hyperactivity in response to the TMT-paired context: role for mGlu3 signaling
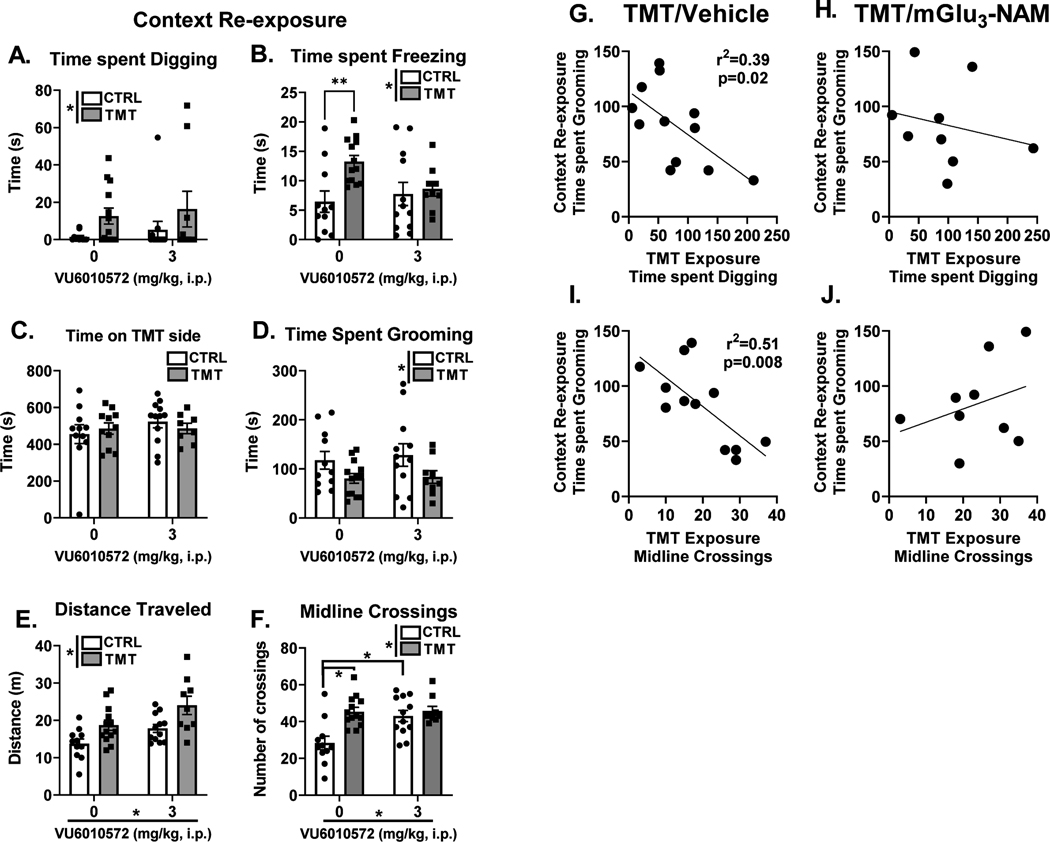
Figure 3 A–F shows the behavioral response during the context re-exposure. For digging (Fig. 3A), a main effect of TMT exposure was found to increase time spent digging (F (1, 41) = 4.58, p=0.038, η2=0.10). For freezing, (Fig. 3B), a main effect of TMT exposure was found (F (1, 41) = 5.70, p=0.022, η2=0.11). No effect of mGlu3-NAM or interaction observed for freezing. Planned comparisons showed an increase in time spent freezing between the water and TMT group in the vehicle condition (p<0.05), but not the mGlu3-NAM condition. No effect of TMT exposure or mGlu3-NAM was observed for time spent on the TMT side (Fig. 3C). For time spent grooming (Fig. 3D), a main effect of TMT showed reduced grooming behavior (F (1, 41) = 5.73, p=0.02, η2=0.12), but no significant main effect of mGlu3-NAM or interaction was observed.
Figure 3. TMT exposure produces freezing and hyperactivity in response to the TMT-paired context: role for mGlu3 signaling during stressor on the freezing response.
During the context re-exposure, a main effect of TMT exposure increased both time spent digging (A) and time spent freezing (B). A freezing response was observed in the control group but not in the mGlu3-NAM group (B). Time spent on the TMT side of the chamber was not affected by TMT exposure or mGlu3-NAM (C). Time spent grooming (D) was decreased as a result of TMT exposure. Distance traveled (E) was increased by the TMT exposure and mGlu3NAM pretreatment. Finally, midline crossings (F) were increased by both the TMT exposure and mGlu3-NAM pretreatment. Time spent digging during the TMT exposure negatively correlated with time spent grooming during the context re-exposure in the TMT/vehicle group (G), but not the TMT/mGlu3-NAM group (H). The number of midline crossings during the TMT exposure negatively correlated with time spent grooming during the context re-exposure in the TMT/vehicle group (I), but not the TMT/mGlu3-NAM group (J). * p≤0.05 significantly different from Control
For distance traveled (Fig. 3E), a main effect of TMT exposure (F (1, 41) = 13.36, p=0.0007, η2=0.22) and mGlu3-NAM (F (1, 41) = 9.57, p=0.004, η2=0.15) were observed, both showing increases in distance traveled. No interaction effect was found. For the number of midline crossings (Fig. 3F), a main effect of TMT exposure (F (1, 41) = 11.15, p=0.0018, η2=0.17), mGlu3-NAM (F (1, 41) = 6.43, p=0.02, η2=0.10) and a TMT x mGlu3-NAM interaction effect was observed (F (1, 41) = 5.77. p=0.02, η2=0.09). The TMT/vehicle group displayed greater midline crossings compared to the control/vehicle group (p=0.0003). Interestingly, this difference was not observed in the animals that received mGlu3-NAM pretreatment (p > 0.05). This lack of effect was likely driven by an increase in midline crossings in the control/mGlu3-NAM group compared to the control/vehicle group (p=0.002). Together, these data indicate that TMT exposure produces freezing and hyperactivity in the previously paired TMT context, and that mGlu3 signaling during the TMT exposure contributes to the freezing response in the context re-exposure.
Understanding individual differences in response to stressor may prove valuable in understanding vulnerability to stress-related disorders [67] and behavioral outcomes in rodents [45, 59]. Therefore, correlational analyses were conducted between stress-reactive behaviors during the TMT exposure (Fig. 2) and behavioral responses to the context re-exposure 2 weeks later (Fig. 3). Interestingly, in the TMT/vehicle group, time spent grooming during the context re-exposure correlated with 3 distinct stress-reactive behaviors during the TMT exposure. First, time spent digging (Fig. 3G) negatively correlated with time spent grooming (r2=0.39, p=0.02). This correlation was not observed in the TMT/mGlu3-NAM group (Fig. 3H). The number of midline crossings during the TMT exposure negatively correlated with time spent grooming during the context re-exposure (r2=0.51, p=0.008) in the TMT/vehicle group (Fig. 3I), but not in the TMT/mGlu3-NAM group (Fig. 3J). These correlations were not observed in either control group (control/vehicle: Digging X Grooming: r=−0.07; Midline Crossings X Grooming: r=−0.11; or the Control/mGlu3-NAM: Digging X Grooming: r=−0.019; Midline Crossings X Grooming: r=−0.16) group. These correlations suggest an association between the degree of stress-reactivity to the TMT exposure with the degree of engagement in grooming behavior during the context re-exposure. Interestingly, these associations were not observed in the TMT-exposed rats that were pretreated with mGlu3-NAM suggesting that the mGlu3-NAM may disrupt this association.
4.4. mGlu3-NAM produces lasting anxiolytic-like behavioral effects
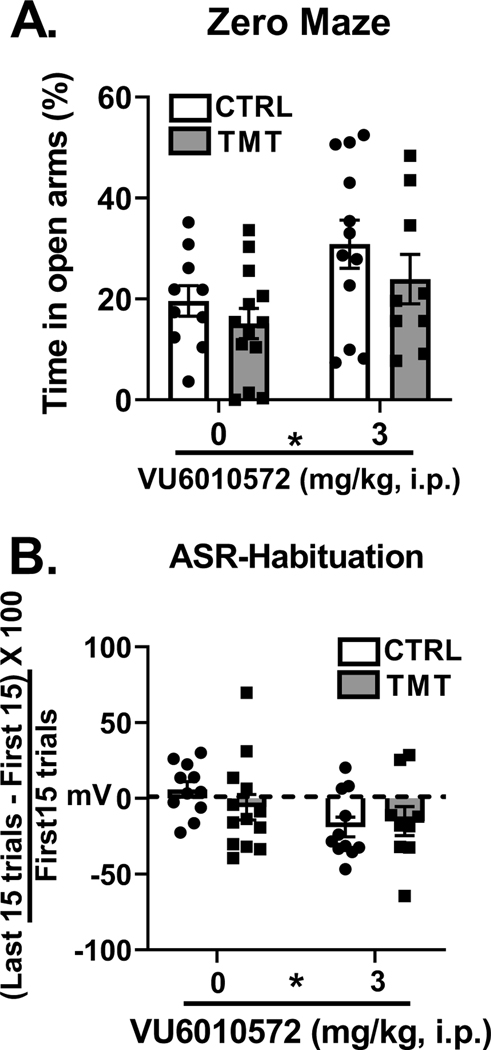
Figure 4 shows analysis of the zero-maze test and acoustic startle response test approximately 2 weeks after TMT exposure and mGlu3-NAM pretreatment. For percent time spent in the open arms of the zero maze (Fig. 4A), a main effect of mGlu3-NAM was found (F (1, 40) = 6.19, p=0.01, η2=0.13), indicating greater percent time spent in the open arms, but no effect of TMT exposure or interaction was observed. Analysis of the total distance traveled in the zero maze showed no effect of TMT exposure or mGlu3-NAM (control/vehicle – 3.97 ± 0.18; control/mGlu3-NAM - 4.19 ± 0.26; TMT/vehicle – 3.85 ± 0.26; TMT/mGlu3-NAM – 4.80 ±0.53). Analysis of the number of entries into the open arm showed no main effect of TMT exposure or mGlu3-NAM (control/vehicle – 10.2 ± 1.67; control/mGlu3-NAM – 12.54 ± 1.63; TMT/vehicle – 7.61 ± 1.75; TMT/mGlu3-NAM – 9.8 ± 1.48). Interestingly, TMT exposure decreased the number of closed arm entries (F (1, 40) = 4.24, p = 0.04, η2=0.09; control/vehicle: 10.3 ± 1.9; control/mGlu3-NAM: 12.6 ± 1.7; TMT/vehicle: 6.84 ± 1,7; TMT/mGlu3-NAM: 8.9 ± 1.4). mGlu3-NAM did not affect the number of closed arm entries. No effect of mGlu3-NAM or TMT exposure was found for total arm entries (control/vehicle: 20.5 ± 3.57; control/mGlu3-NAM: 25.18 ± 3.38; TMT/vehicle: 14.46 ± 3.47; TMT/mGlu3-NAM: 18.7 ± 2.93). In the acoustic startle test, there were no significant effects of TMT exposure or mGlu3- NAM on average peak startle response (mV, control/vehicle - 770.81 ± 128.30; control/ mGlu3 - NAM - 957.31 ± 310.94; TMT/Vehicle - 481.33 ± 83.06; TMT/mGlu3-NAM - 1125.47 ± 404.21). However, mGlu3-NAM treated rats displayed greater habituation (Fig. 4B) across the 30-trials (F (1, 40) = 4.95, p=0.03, η2=0.12), with no significant effect of TMT or interaction. These results indicate that mGlu3-NAM produces lasting anxiolytic-like effects and facilitates habituation to the startle response two weeks after the mGlu3-NAM injection. Changes were not observed as an effect of TMT exposure, but as main effects of mGlu3 -NAM itself, demonstrating that these drug effects are not stressor-related.
Figure 4. mGlu3-NAM produces lasting anxiolytic-like behavioral effects.
mGlu3-NAM treatment 2 weeks before testing resulted in increased time spent in the open arms of the zero-maze test (A) and increased habituation of acoustic startle responses (B). TMT exposure did not affect zero maze or ASR measures. * p≤0.05 significantly different from Control.
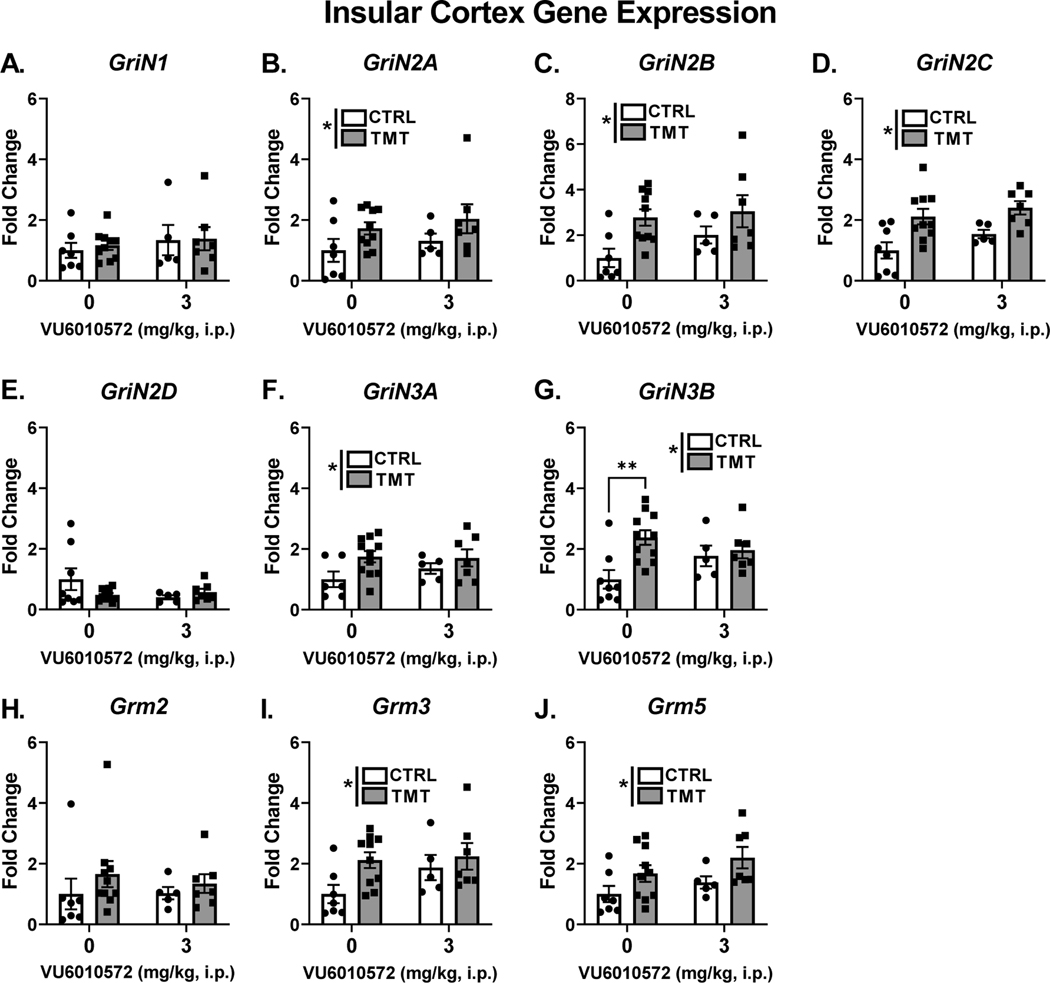
4.5. TMT exposure produces lasting increases in glutamate receptor gene expression in the insular cortex
Figure 5 shows the effects of TMT exposure and mGlu3-NAM on glutamate receptor transcripts in the insular cortex. Neither TMT exposure nor mGlu3-NAM affected the expression of the house-keeper gene (β-actin). For NMDA receptor transcripts, no effect of TMT exposure, mGlu3-NAM or interaction was found for GriN1 expression (Fig. 5A). For GriN2A (Fig. 5B), GriN2B (Fig. 5C), and GriN2C (Fig. 5D), a main effect of TMT exposure showed increased expression (GriN2A - F (1, 25) = 4.41, p = 0.04, η2 = 0.15; GriN2B - F (1, 25) = 8.02, p=0.009, η2 = 0.22; GriN2C - F (1, 26) = 14.50, p=0.0008, η2 = 0.32). There was no main effect of the mGlu3-NAM or interaction. For GriN2D (Fig. 5E), there was no effect of TMT exposure, mGlu3-NAM or interaction. A main effect of TMT exposure showed increased expression of GriN3A (F (1, 25) = 5.09, p=0.03, η2 = 0.16; Fig. 5F), but did not show an effect of the mGlu3-NAM or interaction. GriN3B expression (Fig. 5G) was increased by TMT exposure (F (1, 27) = 6.98, p=0.013, η2 = 0.17), but not affected by mGlu3-NAM, and a significant TMT exposure x mGlu3NAM interaction was observed (F (1, 27) = 4.02, p=0.05, η2 = 0.10). Post-hoc analyses showed that GriN3B expression was increased in the TMT/vehicle group compared to the control/vehicle group (p=0.002), an effect that was prevented by Glu3-NAM pretreatment (i.e., no difference between TMT/ mGlu3-NAM vs. control/mGlu3-NAM groups).
Figure 5. TMT exposure produces lasting increases in glutamate receptor gene expression in the insular cortex.
TMT exposure and mGlu3 -NAM did not affect GriN1 expression (A). TMT exposure increased expression of GriN2A (B), GriN2B (C), and GriN2C (D). TMT exposure and mGlu3-NAM did not affect expression of GriN2D (E). TMT exposure increased expression of GriN3A (F) and GriN3B (G). An interaction effect between TMT exposure and mGlu3-NAM was found for GriN3B, and TMT exposure increased expression in the vehicle-treated group, but not the mGlu3 -NAM group (G). Grm2 expression was not affected by TMT exposure and mGlu3-NAM (H). TMT exposure increased expression of Grm3 (I) and Grm5 (J). * p≤0.05 significantly different from Control.
For mGlu receptor transcripts, Grm2 (Fig. 5H) was not affected by TMT exposure or mGlu3-NAM. Grm3 (Fig. 5I) and Grm5 (Fig. 5J) were upregulated as shown by a main effect of TMT exposure (Grm3 - F (1, 25) = 4.39, p=0.04, η2 = 0.13; Grm5 - F (1, 25) = 6.01, p=0.02, η2 = 0.18) with no effect of the mGlu3-NAM or interaction. These data indicate that TMT exposure increases gene expression of several NMDA and mGlu receptor sub-units in the insular cortex, and that mGlu3 signaling during TMT exposure may mediate the stressor-induced increase of GriN3B.
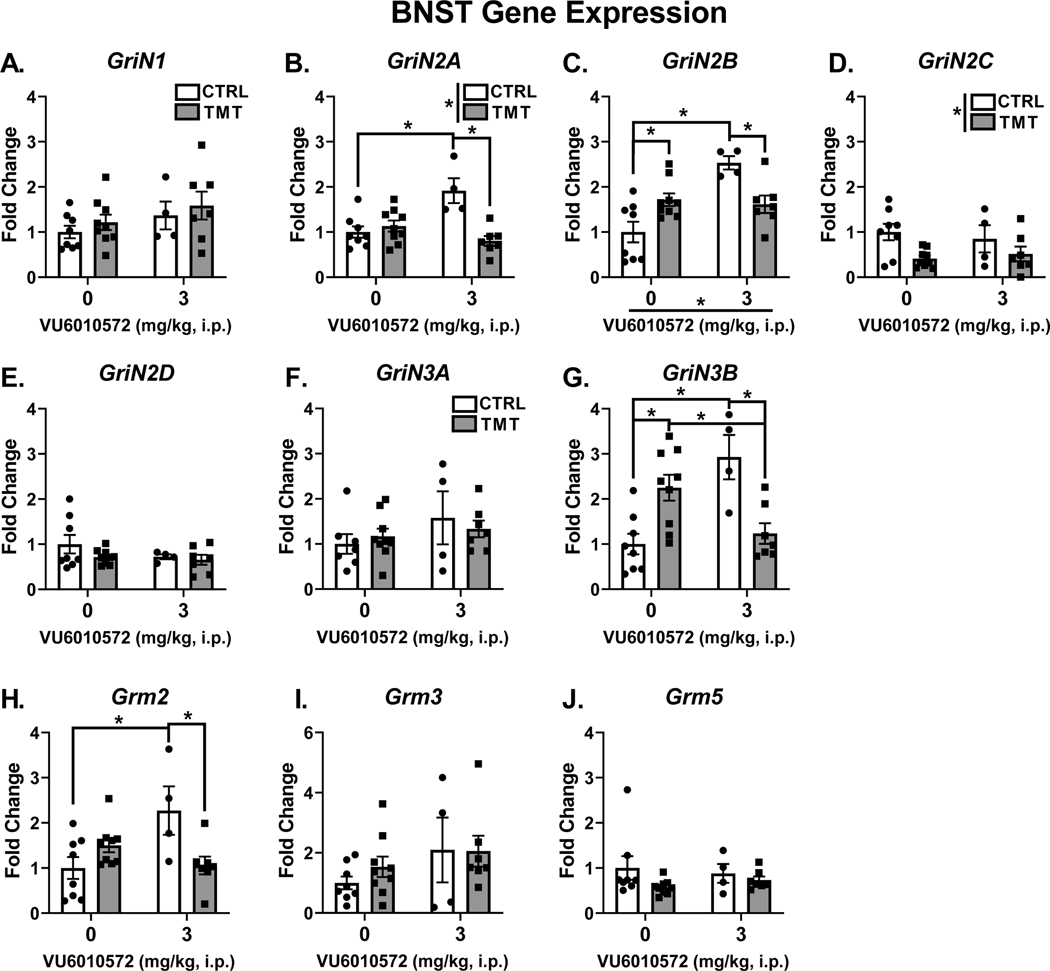
4.6. TMT exposure and mGlu3-NAM produce lasting changes in glutamate receptor transcript levels in the BNST
Figure 6 shows the gene expression changes in the BNST following TMT exposure and mGlu3-NAM pretreatment. Neither TMT exposure nor mGlu3-NAM affected the expression of the house-keeper gene (β-actin). For NMDA receptor, GriN1 (Fig. 6A) showed no effect of TMT or mGlu3-NAM. For GriN2A (Fig. 6B), there was a main effect of TMT exposure (F (1, 24) = 10.79, p=0.003, η2 = 0.23), no main effect of mGlu3-NAM, but a significant interaction effect (F (1, 24) = 17.35, p=0.0003, η2 = 0.36). mGlu3-NAM increased GriN2A expression in controls (p=0.001), but not in the TMT group. GriN2A expression was decreased by TMT exposure in the mGlu3-NAM group (p=0.0002), but not changed in vehicle-treated group. For GriN2B (Fig. 6C), there was a significant main effect of mGlu3-NAM (F (1, 24) = 12.71, p=0.0016, η2 = 0.26), no main effect of TMT exposure, and a significant interaction effect (F (1, 24) = 16.45, p=0.0005, η2 = 0.33). mGlu3-NAM increased expression in controls (p <0.0001) but not in the TMT group. TMT exposure decreased expression in mGlu3-NAM treated rats (p=0.016), but increased expression in vehicle treated rats (p=0.015). GriN2C (Fig. 6D) was downregulated as a main effect of TMT exposure (F (1, 24) = 7.399, p=0.011, η2 = 0.22), but no effect of mGlu3-NAM or significant interaction was found. No effect of TMT exposure, mGlu3-NAM, or interaction was found for GriN2D expression (Fig. 6E) or GriN3A expression (Fig. 6F). For GriN3B (Fig. 6G), no main effect of TMT or mGlu3-NAM was observed, but a significant interaction effect was found (F (1, 24) = 23.75, p < 0.0001, η2 = 0.49). TMT exposure increased expression of GriN3B in the vehicle group (p=0.005), but decreased expression in the mGlu3-NAM group (p=0.0033). mGlu3 -NAM increased Grin3B expression in controls (p=0.0007), but decreased expression in the TMT group (p=0.028).
Figure 6. TMT exposure and mGlu3 -NAM produce lasting changes in glutamate receptor transcript levels in the BNST.
The TMT exposure and mGlu3-NAM did not affect GriN1 (A) gene expression in the BNST. mGlu3-NAM increased expression of GriN2A (B) and GriN2B (C) in the control group only. TMT exposure reduced the expression of GriN2A and GriN2B in the mGlu3-NAM group. For GriN2B, TMT exposure increased expression in the vehicle group, but decreased expression in the mGlu3-NAM group. TMT exposure decreased expression of GriN2C (Fig. 6D). TMT exposure and mGlu3-NAM did not affect GriN2D (E) or GriN3A (F) expression. TMT exposure increased GriN3B (G) expression in the vehicle group, but decreased expression in the mGlu3-NAM group. mGlu3-NAM reversed the stressor-induced upregulation of GriN3B (G). mGlu3-NAM increased GriN3B expression in the vehicle group (G). mGlu3-NAM increased Grm2 (H) expression in the vehicle group, but TMT exposure prevented this effect. TMT exposure and mGlu3-NAM did not affect expression levels of Grm3 (I) and Grm5 (J).
For mGlu receptors, Grm2 (Fig. 6H), no main effect of TMT or mGlu3-NAM was observed, but a significant TMT x mGlu3-NAM interaction was found (F (1, 24) = 11.25, p=0.0026, η2 = 0.31). Post hoc analyses showed that mGlu3-NAM increased Grm2 expression in control rats (p=0.0075) but did not affect the TMT group. TMT exposure decreased Grm2 expression in the mGlu3-NAM group (p=0.012) but did not affect the vehicle group. Grm3 and Grm5 (Fig. 6I and Fig. 6J, respectively) expression were not affected by TMT exposure or mGlu3-NAM.
5. Discussion
These studies show that TMT exposure produces behavioral (context re-exposure) and molecular adaptations (insular cortex and BNST glutamate transcript upregulation) 2 weeks after the exposure, demonstrating lasting behavioral and molecular phenotypes from this stressor model. Furthermore, mGlu3 signaling during exposure to the TMT predator odor stressor contributes to some extent to both behavioral (freezing response during the context reactivity) and molecular (GriN3B upregulation in the insular cortex and BNST) adaptations approximately 2 weeks after the stressor exposure in male rats.
Consistent with previous findings, the TMT exposure produced engagement in stress-reactive behaviors (freezing, digging, avoidance, and decreased grooming, distance traveled and midline crossings), indicating that TMT exposure is an acute stressor [54, 55, 59, 63, 68]. Pretreatment with the mGlu3-NAM (VU6010572) did not affect any of these behaviors, suggesting that mGlu3 signaling during the stressor does not contribute to the engagement of acute stress-reactive behaviors. Prior studies in mice show that a similar mGlu3-NAM (VU0650786) administered prior to restraint stress prevented decreased sucrose intake indicative of motivational behavior caused by the restraint stress [19] and that VU6010572 produced acute anti-depressant-like behavioral effects [32]. Together with the present findings, these data serve to specify the acute effects of mGlu3-NAM, which may be related more to anti-depressant-like effects rather than effects on stress-reactivity.
TMT exposure increased time spent freezing during the context re-exposure test conducted 2 weeks after the stressor, which is consistent with prior reports using TMT exposure in rats [54, 55, 68]. Note that rats engaged in a very small amount of freezing (about 1% time spent freezing during the context re-exposure). As such, TMT exposure did not produce a high degree of freezing behavior during the context re-exposure. Interestingly, during the context re-exposure, rats in the TMT groups also showed increases in measures of locomotion and general activity (increased digging, distance traveled, midline crossings, and decreased grooming), which together with freezing may reflect a hypervigilant behavioral phenotype in response to the context re-exposure in rats previously exposed to TMT. Pretreatment with the mGlu3-NAM VU6010572 before the TMT exposure that occurred 2 weeks earlier prevented the freezing response to the context re-exposure. This indicates that mGlu3 activation as a consequence of the TMT stressor exposure contributes to the formation of a fear-like association with the stressor context. The context re-exposure did not show an avoidance phenotype (i.e., less time spent on the TMT side of the chamber), which indicates that this model does not capture the avoidance symptom cluster observed in PTSD, as some other predator odor models do [45]. However, the absence of an avoidance phenotype is consistent with our prior publications [59, 63, 69], and other published work using TMT exposure [57, 70, 71]. Correlational analyses in the TMT/vehicle group showed that time spent grooming during the context re-exposure negatively correlated with both time spent digging and the number of midline crossings during the TMT exposure. These data patterns suggest a potential association between stress-reactivity during the TMT exposure with the lasting behavioral adaptations in response the context re-exposure (specifically diminished grooming). Interestingly, these correlations in the TMT/vehicle group were not observed in the TMT/mGlu3-NAM group, suggesting that mGlu3-NAM pretreatment prior to stressor disrupted the associations between TMT exposure and some of the context re-exposure stress reactivity measures.
The mGlu3-NAM pretreatment also produced other lasting behavioral changes. While TMT exposure did not affect zero maze and ASR measures, the mGlu3-NAM produced anxiolytic effects on the zero maze and promoted greater habituation of arousal response in the ASR test. Because mGlu3-NAM was administered 2 weeks prior to behavioral tests, these findings suggest a lasting effect of the mGlu3-NAM on anxiety and arousal processes, which may contribute to the therapeutic potential of negative allosteric modulation of mGlu3 as to date VU6010572 has been shown to have acute and rapid anti-depressant-like effects [32]. Further, mGlu2/3 antagonists have been proposed as potential alternative anti-depressants with similar acute and lasting efficacy and mechanisms to ketamine in the absence of abuse liability [17]. Therefore, these novel findings on anxiety-like behavior and arousal habituation show that a single administration of the mGlu3-NAM VU6010572 is sufficient to produce lasting therapeutic effects, and therefore may have potential as a new glutamate-acting therapeutic. Note that these drug effects were not dependent on the stressor condition (i.e., main effect of mGlu3-NAM pretreatment), but were observed in both the control and TMT groups. Finally, while it is possible that the context re-exposure testing affected subsequent zero maze and ASR measures, these tests were separated by 24 hours to minimize this concern.
TMT exposure produced lasting increases of several glutamate receptor transcripts, including mGlu (Grm3, Grm5) and NMDA (GriN2A, GriN2B, GriN2C, GriN3A, GriN3B) in the insular cortex. Because many of these genes encode for synaptic glutamate receptors [72], these data may reflect changes to excitatory synaptic number, density, and/or receptor composition of glutamate receptors that potentially result in functional differences. Because the insular cortex is involved in negative affect, anxiety, fear memories and threat conditioning [34, 37, 38, 44, 73], and NMDA receptor adaptations are involved in synaptic plasticity in the insular cortex [74], these adaptations could in part underlie the hypervigilance and freezing phenotypes observed in the context re-exposure test.
Similar to the insular cortex, the BNST showed increased NMDA receptor transcripts (GluN2B and GluN3B) from TMT exposure. Interestingly, TMT exposure decreased expression of GriN2C. Collectively, these data suggest that TMT exposure may have altered the sub-unit composition of NMDA receptors in the BNST. Surprisingly, TMT exposure also decreased expression of some NMDA receptor transcripts only in the mGlu3-NAM treated group, including GriN2A, GriN2B, GriN3B and Grm2. This effect may have been driven by the fact the mGlu3-NAM increased expression of these transcripts, showing a similar effect as TMT exposure. Together, these data suggest that mGlu3-NAM and TMT exposure affect glutamate receptor transcription. Prior data suggests that mGlu3 receptors may be activated during stressor exposure [19, 63]. Therefore, the upregulation of these NMDA receptor transcripts induced by blocking mGlu3 may be offset by the subsequent activation of mGlu3 induced by the TMT exposure, thus preventing the upregulation of the NMDA receptor transcripts. Indeed, prior studies have also demonstrated involvement of mGlu2/3 signaling in stress-induced adaptations to the BNST. Specifically, mGlu2/3 agonism (LY379268) potentiated foot-shock induced c-Fos increases in the dorsolateral BNST [75].
Interestingly, mGlu3-NAM pretreatment prior to TMT exposure blocked the upregulation of GriN3B (encodes GluN3B) induced by TMT exposure in both the insular cortex and the BNST. GluN3B is an NMDA receptor subunit expressed at low levels in the brain [76] but may play an important role in the lasting alterations from synaptic plasticity and synaptogenesis [77]. In contrast to canonical NMDA receptors (GluN1-GluN2 heterotetramers), GluN3-containing NMDA receptors (GluN1-GluN3A/B) have a smaller single channel conductance, lower Ca2+ permeability, less sensitivity to the Mg2+ block, and are located outside of the synapse [77].
Early studies indicated that the GluN3 subunits diminished glutamate-evoked NMDA currents [78], which was proposed to indicate a dominant-negative function of GluN3B on traditional GluN1-GluN2 currents. Therefore, these data could indicate that mGlu3 signaling during the TMT predator odor stressor diminishes synaptic/phasic NMDA receptor function through sequestration of glutamate by the dominant-negative sub-unit GluN3B. Interestingly, GluN1-GluN3 heterotetramers are not sensitive to glutamate or NMDA, but all 4 subunits bind glycine [77]. Because endogenous concentrations of glycine constitutively occupy these receptors, it has been proposed that GluN1/GluN3 heterotetramers remain open in vivo and serve to modulate tonic excitation [77, 79]. Therefore, the upregulation of GriN3B transcript expression in the insular cortex and BNST by stressor-induced mGlu3 signaling might also reflect enhanced tonic excitation in these brain regions as a lasting adaptation to stressor. As such, important future directions will examine how these adaptations affect cellular physiology. Importantly, these findings are the first to show that TMT exposure produces lasting adaptations to excitatory gene expression in the insular cortex and BNST, and that mGlu3 signaling during stressor mediates GriN3B transcriptional upregulation in both brain regions. Finally, given recent clinical findings implicating GriN3B expression levels as a predictive blood biomarker of PTSD outcomes following trauma [80], the mGlu3-NAM VU6010572 may have potential therapeutic effects by attenuating stressor-induced GriN3B expression in the brain.
The insular cortex and BNST form reciprocal projections [81, 82] and both regions contribute to affective behavioral measures in rodents [37, 38, 83] and humans [34]. Recent studies have shown that insular cortex/BNST circuitry are involved in negative affective measures associated with alcohol withdrawal in rodents [44]. Therefore, these findings showing stressor- and mGlu3-NAM-induced adaptations to glutamate receptors in the insular cortex and BNST could reflect adaptations to insular cortex/BNST excitatory circuitry as a result of stressor and mGlu3-NAM, and further implicate this circuitry in stress-induced changes. Future work will investigate the functional involvement of insular cortex/BNST circuitry on lasting behavioral adaptations of the TMT exposure (i.e. context re-exposure hyperactivity, freezing and anxiolytic effects of mGlu3-NAM).
Because glutamatergic signaling is involved in learning and fear-memory associations [84], these data may demonstrate that mGlu3-NAM disrupted the formation of the stress/context association through its effects on NMDA receptor transcriptional regulation. mGlu3 receptors have specifically been implicated in learning and cognition [85], further implicating mGlu3 receptors in the learning or formation of stressor environments. mGlu2/3 activation potentiated NMDAR-currents in isolated hippocampal CA1 neurons [86]. Specific SNPs of the Grm3 gene are associated with cognitive and working memory deficits in schizophrenia [87]. mGlu3 may also have neuroprotective properties, showing that group II agonism protects against NMDAR-induced excitotoxic degeneration in cultured neurons [88]. Based on these studies demonstrating a role for mGlu3 signaling in learning, cognition, and NMDA receptor function, the mGlu3-NAM pretreatment may have partially disrupted the formation of the fear memory associated with the stressor context through its effects on NMDA receptors.
An important consideration for the present findings is that like the TMT groups, the control groups experienced exposure to a novel environment (although in the absence of TMT). This experience was potentially a stressor and therefore, this feature of our experimental design should be considered when making conclusions about the effects of mGlu3-NAM and the observed adaptations in behavior and gene expression. Furthermore, the effects of TMT exposure and mGlu3 signaling were not evaluated in female rats, which is a limitation to these studies as sex differences may exist in response to TMT stressor and mGlu3-NAM [59]. Furthermore, the BNST is sexually dimorphic [89], suggesting the possibility of sex differences in the gene expression changes in the BNST specifically. Important follow-up studies will evaluate these effects in female rats. Finally, blockade of mGlu3 receptors by negative allosteric modulation was chosen for these experiments based on the hypothesis that stressor activates mGlu3 receptors, resulting in plasticity that has lasting effects [19, 20, 63]. However, it will also be important to evaluate the direct role of pharmacological mGlu3 activation in the acute and lasting effects of TMT exposure for a more comprehensive evaluation of mGlu3 signaling in the lasting effects of stress. The importance of evaluating mGlu3 activation in stressor-related adaptations is reflected by work showing that mGlu2/3 activation by LY354740 produces anxiolytic effects on the elevated plus-maze (EPM) test and blocks the EPM-induced c-Fos expression in limbic brain regions [90]. This previous work suggests the possibility that mGlu3 activation might attenuate the acute stress response to the TMT exposure.
These findings show that mGlu3-NAM did not affect acute stress-reactivity in response to TMT exposure but did contribute to lasting phenotypes induced by the TMT exposure. TMT exposure produced a hypervigilant and freezing phenotype in response to the context re-exposure 2 weeks later, and mGlu3-NAM pretreatment prevented the freezing response to the context re-exposure. Gene expression analysis show that TMT exposure produced adaptations to glutamate receptors in the insular cortex and BNST, and that mGlu3-NAM during stressor mediated the transcriptional upregulation of GriN3B in the insular cortex and BNST. Finally, mGlu3-NAM produced lasting anxiolytic-like behavioral phenotypes in both control and TMT groups. These findings show that mGlu3 receptors are involved in some of lasting adaptations to stressor and may reflect a promising therapeutic target for neuropsychiatric disorders.
Highlights.
mGlu3-NAM pretreatment does not affect TMT exposure stress-reactive behaviors.
TMT exposure produces freezing and hyperactivity in response to the TMT-paired context.
mGlu3-NAM pretreatment prevents freezing response to the context.
mGlu3-NAM pretreatment produces lasting anxiolytic-like effects.
TMT exposure and mGlu3-NAM affect glutamatergic gene expression in the insular cortex and BNST.
Acknowledgements:
This work was supported in part by the National Institute of Health AA026537 and AA011605 (JB) and by the Bowles Center for Alcohol Studies. RET and MB were supported in part by NS007431.
Footnotes
Ryan E. Tyler: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Data Curation, Writing – Original draft, Writing – Review and editing, Supervision, Project Administration. Maya N. Bluitt: Methodology, Validation, Formal Analysis, Investigation, Data Curation. Julie L. Engers: Methodology, Validation. Craig W. Lindsley: Methodology, Validation, Writing – Review and editing, Resources. Joyce Besheer: Conceptualization, Methodology, Resources, Writing – Review and editing, Supervision, Project Administration, Funding Acquisition.
Data Availability Statement: Data available on request from the authors.
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Averill LA, et al. , Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett, 2017. 649: p. 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MT, et al. , In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proceedings of the National Academy of Sciences, 2019. 116(23): p. 11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes SE, et al. , Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc Natl Acad Sci U S A, 2017. 114(31): p. 8390–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumeister A, et al. , Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry, 2013. 18(9): p. 1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishi D, et al. , Glutamatergic system abnormalities in posttraumatic stress disorder. Psychopharmacology (Berl), 2015. 232(23): p. 4261–8. [DOI] [PubMed] [Google Scholar]
- 6.Joffe ME, et al. , Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies. ACS Chem Neurosci, 2018. 9(9): p. 2188–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popoli M, et al. , The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience, 2011. 13(1): p. 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowy MT, Gault L, and Yamamoto BK, Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem, 1993. 61(5): p. 1957–60. [DOI] [PubMed] [Google Scholar]
- 9.Lowy MT, Wittenberg L, and Yamamoto BK, Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem, 1995. 65(1): p. 268–74. [DOI] [PubMed] [Google Scholar]
- 10.Moghaddam B, Stress Preferentially Increases Extraneuronal Levels of Excitatory Amino Acids in the Prefrontal Cortex: Comparison to Hippocampus and Basal Ganglia. Journal of Neurochemistry, 1993. 60(5): p. 1650–1657. [DOI] [PubMed] [Google Scholar]
- 11.Musazzi L, et al. , Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One, 2010. 5(1): p. e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reznikov LR, et al. , Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci, 2007. 25(10): p. 3109–14. [DOI] [PubMed] [Google Scholar]
- 13.Venero C. and Borrell J, Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci, 1999. 11(7): p. 2465–73. [DOI] [PubMed] [Google Scholar]
- 14.Hill MN, et al. , Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci, 2011. 31(29): p. 10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen EY, et al. , Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A, 2009. 106(33): p. 14075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumoto K, Iijima M, and Chaki S, The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology, 2016. 41(4): p. 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaki S, mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends in Pharmacological Sciences, 2017. 38(6): p. 569–580. [DOI] [PubMed] [Google Scholar]
- 18.Levey DF, et al. , Genetic risk prediction and neurobiological understanding of alcoholism. Translational Psychiatry, 2014. 4(5): p. e391–e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffe ME, et al. , Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry, 2019. 24(6): p. 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker AG, et al. , Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proceedings of the National Academy of Sciences, 2015. 112(4): p. 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biber K, et al. , Expression and Signaling of Group I Metabotropic Glutamate Receptors in Astrocytes and Microglia. Journal of Neurochemistry, 1999. 72(4): p. 1671–1680. [DOI] [PubMed] [Google Scholar]
- 22.Chaki S, et al. , MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology, 2004. 46(4): p. 457–67. [DOI] [PubMed] [Google Scholar]
- 23.Ago Y, et al. , Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology, 2013. 65: p. 29–38. [DOI] [PubMed] [Google Scholar]
- 24.Feyissa AM, et al. , Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry, 2010. 34(2): p. 279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witkin JM, mGlu2/3 receptor antagonism: A mechanism to induce rapid antidepressant effects without ketamine-associated side-effects. Pharmacology Biochemistry and Behavior, 2020. 190: p. 172854. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimizu T, et al. , An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl), 2006. 186(4): p. 587–93. [DOI] [PubMed] [Google Scholar]
- 27.Joffe ME, et al. , mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron, 2020. 105(1): p. 46–59.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reagan LP, et al. , The antidepressant agomelatine inhibits stress-mediated changes in amino acid efflux in the rat hippocampus and amygdala. Brain Research, 2012. 1466: p. 91–98. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS, et al. , The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry, 2010. 15(3): p. 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grillo CA, et al. , Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience, 2015. 284: p. 430–443. [DOI] [PubMed] [Google Scholar]
- 31.Engers JL, et al. , Discovery of a Selective and CNS Penetrant Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 3 with Antidepressant and Anxiolytic Activity in Rodents. Journal of Medicinal Chemistry, 2015. 58(18): p. 7485–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engers JL, et al. , Design and Synthesis of N-Aryl Phenoxyethoxy Pyridinones as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Medicinal Chemistry Letters, 2017. 8(9): p. 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christianson JP, et al. , Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry, 2011. 70(5): p. 458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez RP, et al. , Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Translational Psychiatry, 2015. 5(6): p. e591–e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig AD, Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 2003. 13(4): p. 500–505. [DOI] [PubMed] [Google Scholar]
- 36.Craig AD, How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci, 2009. 10(1): p. 59–70. [DOI] [PubMed] [Google Scholar]
- 37.Méndez-Ruette M, et al. , The Role of the Rodent Insula in Anxiety. Frontiers in Physiology, 2019. 10(330). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez M, et al. , Interoceptive Insular Cortex Mediates Both Innate Fear and Contextual Threat Conditioning to Predator Odor. Front Behav Neurosci, 2019. 13: p. 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai M, Kishi K, and Kato S, Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry, 2007. 22(6): p. 387–94. [DOI] [PubMed] [Google Scholar]
- 40.Lebow MA and Chen A, Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry, 2016. 21(4): p. 450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flanigan ME and Kash TL, Coordination of social behaviors by the bed nucleus of the stria terminalis. Eur J Neurosci, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fetterly TL, et al. , α(2A)-Adrenergic Receptor Activation Decreases Parabrachial Nucleus Excitatory Drive onto BNST CRF Neurons and Reduces Their Activity In Vivo. J Neurosci, 2019. 39(3): p. 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fendt M, Endres T, and Apfelbach R, Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci, 2003. 23(1): p. 23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centanni SW, et al. , Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology, 2019. 44(3): p. 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albrechet-Souza L. and Gilpin NW, The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol, 2019. 30(2 and 3-Spec Issue): p. 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brodnik ZD, et al. , Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine. Neuropharmacology, 2017. 125: p. 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen H, et al. , The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci, 2006. 1071: p. 335–50. [DOI] [PubMed] [Google Scholar]
- 48.Deslauriers J, et al. , Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol Psychiatry, 2018. 83(10): p. 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dielenberg RA and McGregor IS, Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev, 2001. 25(7–8): p. 597–609. [DOI] [PubMed] [Google Scholar]
- 50.Endres T. and Fendt M, Aversion- <em>vs</em> fear-inducing properties of 2,4,5-trimethyl-3-thiazoline, a component of fox odor, in comparison with those of butyric acid. The Journal of Experimental Biology, 2009. 212(15): p. 2324. [DOI] [PubMed] [Google Scholar]
- 51.Endres T. and Fendt M, Aversion- vs fear-inducing properties of 2,4,5-trimethyl-3-thiazoline, a component of fox odor, in comparison with those of butyric acid. J Exp Biol, 2009. 212(Pt 15): p. 2324–7. [DOI] [PubMed] [Google Scholar]
- 52.Hwa LS, et al. , Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacology, 2019. 44(4): p. 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Rosen JB, Asok A, and Chakraborty T, The smell of fear: innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Frontiers in neuroscience, 2015. 9: p. 292–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwendt M, et al. , A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Translational Psychiatry, 2018. 8(1): p. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shallcross J, et al. , The Divergent Effects of CDPPB and Cannabidiol on Fear Extinction and Anxiety in a Predator Scent Stress Model of PTSD in Rats. Frontiers in behavioral neuroscience, 2019. 13: p. 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas RM, Urban JH, and Peterson DA, Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol, 2006. 201(2): p. 308–15. [DOI] [PubMed] [Google Scholar]
- 57.Wallace KJ and Rosen JB, Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci, 2000. 114(5): p. 912–22. [DOI] [PubMed] [Google Scholar]
- 58.Whitaker AM, Gilpin NW, and Edwards S, Animal models of post-traumatic stress disorder and recent neurobiological insights. Behavioural pharmacology, 2014. 25(5–6): p. 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ornelas LC, et al. , Increased alcohol self-administration following exposure to the predator odor TMT in active coping female rats. Behav Brain Res, 2020: p. 113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makhijani VH, et al. , The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats. Psychopharmacology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrero JP, et al. , Mu opioid receptor regulation of glutamate efflux in the central amygdala in response to predator odor. Neurobiology of Stress, 2019. 11: p. 100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adamec R, Blundell J, and Burton P, Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav, 2005. 86(1–2): p. 75–91. [DOI] [PubMed] [Google Scholar]
- 63.Tyler RE, et al. , Exposure to the predator odor TMT induces early and late differential gene expression related to stress and excitatory synaptic function throughout the brain in male rats. Genes, Brain and Behavior, 2020. n/a(n/a): p. e12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paxinos G. and Watson C, The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. 2006: Elsevier Science. [Google Scholar]
- 65.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001. 25(4): p. 402–8. [DOI] [PubMed] [Google Scholar]
- 66.Richardson JTE, Eta squared and partial eta squared as measures of effect size in educational research. Educational Research Review, 2011. 6(2): p. 135–147. [Google Scholar]
- 67.Yehuda R, et al. , Post-traumatic stress disorder. Nat Rev Dis Primers, 2015. 1: p. 15057. [DOI] [PubMed] [Google Scholar]
- 68.Rosen JB, West EA, and Donley MP, Not all rat strains are equal: differential unconditioned fear responses to the synthetic fox odor 2,4,5-trimethylthiazoline in three outbred rat strains. Behav Neurosci, 2006. 120(2): p. 290–7. [DOI] [PubMed] [Google Scholar]
- 69.Ornelas LC, Van Voorhies K, and Besheer J, The role of the nucleus reuniens in regulating contextual conditioning with the predator odor TMT in female rats. Psychopharmacology, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGregor IS, et al. , Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res, 2002. 129(1–2): p. 1–16. [DOI] [PubMed] [Google Scholar]
- 71.Blanchard DC, et al. , Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci, 2003. 117(2): p. 360–8. [DOI] [PubMed] [Google Scholar]
- 72.Ehlers MD, Synapse structure: Glutamate receptors connected by the shanks. Current Biology, 1999. 9(22): p. R848–R850. [DOI] [PubMed] [Google Scholar]
- 73.Casanova JP, et al. , A role for the interoceptive insular cortex in the consolidation of learned fear. Behav Brain Res, 2016. 296: p. 70–77. [DOI] [PubMed] [Google Scholar]
- 74.Parkes SL, et al. , Differential role of insular cortex muscarinic and NMDA receptors in one-trial appetitive taste learning. Neurobiology of Learning and Memory, 2014. 116: p. 112–116. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Y, et al. , Activation of Group II Metabotropic Glutamate Receptors Attenuates Both Stress and Cue-Induced Ethanol-Seeking and Modulates c-<em>fos</em> Expression in the Hippocampus and Amygdala. The Journal of Neuroscience, 2006. 26(39): p. 9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsuda K, et al. , Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res, 2002. 100(1–2): p. 43–52. [DOI] [PubMed] [Google Scholar]
- 77.Pachernegg S, Strutz-Seebohm N, and Hollmann M, GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends in Neurosciences, 2012. 35(4): p. 240–249. [DOI] [PubMed] [Google Scholar]
- 78.Henson MA, et al. , Influence of the NR3A subunit on NMDA receptor functions. Progress in neurobiology, 2010. 91(1): p. 23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatterton JE, et al. , Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature, 2002. 415(6873): p. 793–798. [DOI] [PubMed] [Google Scholar]
- 80.Lori A, et al. , Transcriptome-wide association study of post-trauma symptom trajectories identified GRIN3B as a potential biomarker for PTSD development. Neuropsychopharmacology, 2021. 46(10): p. 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reichard RA, et al. , Abundant collateralization of temporal lobe projections to the accumbens, bed nucleus of stria terminalis, central amygdala and lateral septum. Brain Structure and Function, 2017. 222(4): p. 1971–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flook EA, et al. , BNST-insula structural connectivity in humans. NeuroImage, 2020. 210: p. 116555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christianson JP, et al. , Safety Signals Mitigate the Consequences of Uncontrollable Stress Via a Circuit Involving the Sensory Insular Cortex and Bed Nucleus of the Stria Terminalis. Biological Psychiatry, 2011. 70(5): p. 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maren S, Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci, 2001. 24: p. 897–931. [DOI] [PubMed] [Google Scholar]
- 85.Maksymetz J, Moran SP, and Conn PJ, Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Molecular Brain, 2017. 10(1): p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trepanier C, et al. , Group II metabotropic glutamate receptors modify N-methyl-D-aspartate receptors via Src kinase. Scientific Reports, 2013. 3(1): p. 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harrison PJ, et al. , The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol, 2008. 22(3): p. 308–22. [DOI] [PubMed] [Google Scholar]
- 88.Bruno V, et al. , The neuroprotective activity of group-II metabotropic glutamate receptors requires new protein synthesis and involves a glial-neuronal signaling. J Neurosci, 1997. 17(6): p. 1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uchida K, et al. , Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biology of Sex Differences, 2019. 10(1): p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Linden AM, et al. , Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology, 2004. 29(3): p. 502–13. [DOI] [PubMed] [Google Scholar]