Abstract
This study was conducted to investigate the effect of anhydrous betaine and hydrochloride betaine on growth performance, meat quality, relaxometry, postmortem glycolysis, and antioxidant capacity of partridge shank broiler chickens. A total of 400 one-day-old male broilers were randomly divided into 5 treatments and fed basal diets supplemented with 0 (control), 500 (L-AB) or 1,000 (H-AB) mg/kg anhydrous betaine, and 642.23 (L-HB) or 1,284.46 (H-HB) mg/kg hydrochloride betaine, respectively. Compared with the control group, anhydrous betaine supplementation significantly increased (P < 0.05) average daily gain and decreased (P < 0.05) drip loss24h in breast and thigh muscles of broilers. The H-AB group further increased (P < 0.05) breast muscle yield, pH24h, immobile water proportion (P21), the contents of crude protein and glutathione (GSH), the activities of creatine kinase (CK) and glutathione peroxidase (GPX), the mRNA expressions of glucose transporter 4 (GLUT4), protein kinase AMP-activated non-catalytic subunit gamma 3 (PRKAG3), nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1) in breast muscle, and a*45min, GLUT4 mRNA expression in thigh muscle, and decreased (P < 0.05) drip loss48h, free water proportion (P22), the contents of lactate and malondialdehyde (MDA) in breast muscle. Moreover, the H-HB group significantly increased (P < 0.05) pH24h, P21 proportion, the activities of CK, total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), and GPX, the content of GSH, the mRNA levels of Nrf2, HO-1, GPX, and γ-glutamate-cysteine ligase catalytic subunit (γ-GCLc) in breast muscle, and the activity and mRNA expression of GPX in thigh muscle, and decreased (P < 0.05) drip loss24h, P22 proportion in breast muscle, and MDA content in breast and thigh muscles. In conclusion, anhydrous betaine showed better effects than hydrochloride betaine in improving growth performance and breast muscle yield of broilers. Moreover, anhydrous betaine (1,000 mg/kg) or equimolar hydrochloride betaine supplementation could improve meat quality by decreasing drip loss, free water proportion, and lactate content, and enhancing muscle antioxidant capacity.
Key words: betaine, broiler, meat quality, anaerobic glycolysis, antioxidant capacity
INTRODUCTION
With the increasing consumption of chicken meat, intensive farming methods have been introduced into broilers production to improve productivity. However, this intensive production may bring some negative effects on broilers, such as poor welfare, decreased meat quality, and flavor (Petracci et al., 2009). The corresponding contradiction is that consumers demand for better quality and taste meat products with the improving living standards. Therefore, a variety of nutritional strategies have been tried to improve meat quality of broilers, in which betaine receives considerable attention due to its nutritional and physiological functions (Attia et al., 2009; Attia et al., 2016; Dong et al., 2020). Betaine, as a methyl donor, plays an important role in regulating osmotic balance, nutrient metabolism, and antioxidant capacity of broilers (Alirezaei et al., 2012; Eklund et al., 2005; Attia et al., 2018). Common sources of betaine are sugar beets and their by-products, such as molasses. Nevertheless, betaine is also available as a feed additive in chemically purified form, and the most popular forms of feed-grade betaine are anhydrous betaine and hydrochloride betaine. Due to different molecular structures, anhydrous betaine shows higher solubility in water when compared with hydrochloride betaine, thereby increasing its osmotic capacity. Hydrochloride betaine induces the pH decline in the stomach, thereby potentially affecting nutrient digestibility in a mode different from anhydrous betaine (Eklund et al., 2005). However, few studies have compared the effect of these 2 betaine products in broilers.
Meat quality can be defined as a set of properties that together identify what we appreciate about meat, which includes color, pH, water-holding capacity (WHC), texture, nutrient composition, and flavor (Duclos et al., 2007; Grashorn, 2010). It had been reported that betaine had positive effects on meat quality by improving muscle WHC (Matthews et al., 2001; Attia et al., 2009; Dong et al., 2020). The water in fresh meat is mainly divided into 3 parts: bound water, immobilized water, and free water, in which the proportions of immobilized water and free water directly affect muscle WHC (Bertram et al., 2002). As an important organic osmolyte, betaine can protect the cell against dehydration and increase water retention to maintain water balance (Eklund et al., 2005; Hoffman et al., 2009). Thus, we hypothesize that betaine may affect water distribution of meat, thereby enhancing muscle WHC of broilers. In addition, muscle postmortem anaerobic glycolysis and antioxidant capacity are widely believed to be 2 important indicators affecting meat quality (Duclos et al., 2007; Alirezaei et al., 2012; Wang et al., 2020). After bleeding, the cessation of oxygen supply changes muscular metabolism. Then, anaerobic glycolysis inevitably occurs and drives lactic acid accumulation. If the ultimate pH of meat is abnormally low caused by extended glycolysis, it will lead to a diminished WHC and poor meat quality (Stephens et al., 2008). Previous studies proved that betaine supplementation could decrease muscle lactate accumulation of horses and regulate muscle anaerobic glycolysis of transported broilers, which indicated that betaine might affect muscle postmortem metabolism of broilers (Warren et al., 1999; Chen et al., 2020). Moreover, lipid peroxidation is a major deterioration reaction in meat which often causes texture and flavor problems (Salih et al., 1987; Fernandez et al., 1997). Chicken meat contains relative abundant polyunsaturated fatty acids, making it susceptible to oxidation (Arshad et al., 2011). Nevertheless, betaine has been confirmed to be a promising antioxidant agent for improving meat quality of broilers under various stress conditions (Attia et al., 2009; Alirezaei et al., 2012; Wen et al., 2019). However, to our knowledge, whether betaine can regulate muscle postmortem glycolysis and antioxidant capacity under a normal environment to improve the meat quality of broilers has not been reported. Therefore, the aim of this study was to investigate the effect of 2 forms of betaines (anhydrous betaine and hydrochloride betaine) on growth performance, meat quality, texture, nutrient composition, relaxometry, postmortem glycolysis, and antioxidant capacity of broilers.
MATERIALS AND METHODS
Animals, Experimental Design, and Diets
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 400 newly hatched male broiler chickens (a Chinese local breed with partridge shank) were randomly divided into 5 groups with 8 replicates of 10 broilers for a 52-d feeding trial. Broilers in the control group were fed a corn-soybean meal basal diet, and those in the other 4 experimental groups were fed the basal diet supplemented with 500 (L-AB, as a low-dose group) or 1,000 (H-AB, as a high-dose group) mg/kg anhydrous betaine, and 642.23 (L-HB, as a low-dose group) or 1,284.46 (H-HB, as a high-dose group) mg/kg hydrochloride betaine, respectively. The levels of anhydrous betaine were selected according to our previous studies (Chen et al., 2018, 2020), and the additions of hydrochloride betaine were calculated according to the content and molecular formula of 2 betaine products to ensure that the betaine component in the hydrochloride betaine group was consistent with that in the anhydrous betaine group (in equimolar). Anhydrous betaine (96%; analyzed value 96.08%) and hydrochloride betaine (98%; analyzed value 98.05%) were obtained from Yixing Skystone Feed Co., Ltd. (Yixing, Jiangsu, China). Broilers consumed mash feed and clean water ad libitum in a temperature-controlled room with a 23 h light: 1 h dark lighting program. The room temperature was maintained at 32 to 34°C for the first 3 d and then gradually reduced by 3°C per week to a final temperature of 20°C. The ingredient compositions and nutrient levels of the basal diet were presented in Table 1. At 52 d of age, broilers were weighed after a 12-h feed withdrawal, and feed intake was recorded by replicate (cage) to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Mortality included in the calculation of FCR was also recorded.
Table 1.
Composition and nutrient contents of basal diets (as-fed basis).
| Items | 1–21 d | 22–42 d | 43–52 d |
|---|---|---|---|
| Ingredients (%) | |||
| Corn | 56.50 | 61.20 | 65.90 |
| Soybean meal | 32.20 | 26.00 | 23.40 |
| Corn gluten meal | 3.70 | 4.20 | 2.00 |
| Soybean oil | 2.61 | 3.87 | 3.97 |
| Dicalcium phosphate | 2.00 | 1.60 | 1.60 |
| Limestone | 1.20 | 1.40 | 1.40 |
| DL-Methionine | 0.15 | 0.08 | 0.08 |
| L-Lysine·HCl | 0.34 | 0.35 | 0.35 |
| Sodium chloride | 0.30 | 0.30 | 0.30 |
| Premix1 | 1.00 | 1.00 | 1.00 |
| Calculated nutrient contents | |||
| Metabolizable energy (MJ/kg) | 12.43 | 12.98 | 13.06 |
| Crude protein (%) | 21.78 | 19.73 | 17.67 |
| Lysine (%) | 1.23 | 1.10 | 1.03 |
| Methionine (%) | 0.51 | 0.42 | 0.38 |
| Total sulfur amino acids (%) | 0.87 | 0.75 | 0.68 |
| Calcium (%) | 1.00 | 0.96 | 0.95 |
| Available phosphorus (%) | 0.46 | 0.39 | 0.39 |
| Analyzed nutrient contents | |||
| Gross energy (MJ/kg) | 16.08 | 16.72 | 16.86 |
| Crude protein (%) | 21.68 | 19.53 | 17.57 |
| Lysine (%) | 1.22 | 1.09 | 1.02 |
| Methionine (%) | 0.52 | 0.42 | 0.37 |
| Total sulfur amino acids (%) | 0.88 | 0.74 | 0.67 |
| Calcium (%) | 1.02 | 0.97 | 0.96 |
| Total phosphorus (%) | 0.68 | 0.61 | 0.60 |
The premix provided per kilogram of diet: vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol acetate), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 65 mg; I (from calcium iodate), 1.1 mg; and Se (from sodium selenite), 0.3 mg.
Sample Collection
One broiler, close to mean weight of birds in each cage, was selected, weighed, and sacrificed by cervical dislocation. After removal of blood and feathers, the hot carcasses were weighed to calculate dressing yield. The eviscerated yield was measured by removing the head, feet, abdominal fat pad, and all of viscera except the lungs and kidneys. The whole breast muscle and thigh muscle were weighed to calculate muscle yields based on eviscerated yield (g/kg). Then, the entire right pectoralis major muscle and tibialis anterior muscle were collected and stored at 4°C for determination of meat quality, texture, nutrient composition, and relaxometry, and a part of left pectoralis major muscle and tibialis anterior muscle (5.0 g) were also immediately taken and stored in liquid nitrogen for other parameters analyses.
Meat Quality Assay
Meat color, including lightness (L*), redness (a*), and yellowness (b*), was measured at 45 min and 24 h postmortem with a colorimeter (Minolta CR-400; Konica Minolta, Tokyo, Japan) using the CIELAB trichromatic system. Muscle pH was assayed at 1 cm depth using a pH meter (HI9125; Hanna Instruments, Padova, Italy) at 45 min and 24 h after slaughter. All measurements were completed in triplicate at 3 different places around the meat sample, and the average values were obtained. The WHC of meat was estimated by determining drip loss and cooking loss as described by Wang et al. (2013). Briefly, the muscle samples were weighed, hung in a sealed ziplock bag, and stored at 4°C. After 24 h and 48 h, the samples were wiped with absorbent paper and weighed again to calculate drip loss based on initial muscle weight. The cooking loss was measured at 24 h after slaughter. The samples were weighed, placed in a sealed ziplock bag, and heated in a water bath until the internal temperature reached 75°C. After cooling to room temperature under running water, the samples were weighed again. Cooking loss was calculated as the percentage of weight loss after cooking.
Muscle Texture Analysis
Meat sample was cut into small pieces (15 mm × 15 mm × 10 mm) and analyzed by texture analyzer (TAXT2i; Stable Micro Systems Corporation, Godalming, UK). The test parameters were set as follows: operation mode, texture profile analysis; probe model, P50; pre-test speed, 2.0 mm/s; test speed, 1.0 mm/s; post-test speed, 2.0 mm/s; target mode, strain (50%); trigger force, 5 g. The texture characteristics include: hardness, springness, cohesiveness, gumminess, chewiness, and resilience.
Muscle Nutrient Composition Analysis
The moisture, crude protein, and ether extract contents of muscle samples were analyzed according to the AOAC (2000) methods. Moisture content (950.46) was determined by weight loss after 12 h at 105°C in an oven, crude protein (981.10) content using the Kjeldahl method and ether extract content (945.16) by the Soxhlet extraction. The data were presented as a proportion of raw meat.
Muscle Nuclear Magnetic Resonance Transverse Relaxation Measurement
The muscle transverse relaxation times (T2) and their corresponding water proportions (P2) were analyzed by low-field nuclear magnetic resonance (NMR) spin−spin relaxation, according to the method by Zhang et al. (2017). Briefly, 2.0 g muscle sample was put into a cylindrical glass tube and then inserted in the NMR probe for testing, using the PQ-001 Niumag Pulsed NMR analyzer (Niumag Electric Corporation, Shanghai, China). The analyzer was operated at a resonance frequency of 22.6 MHz at 32°C. The transverse relaxation was measured using the Carr-Purcell-Meiboom-Gill (CPMG) sequence with a τ-value of 150 μs. Data from 4,096 echoes were acquired as 32 scan repetitions for 1 s under the MultiExp Inv Analysis program (Niumag Electric Corporation) with a multiexponential model.
Muscle Glycolysis and Antioxidant Capacity Measurements
Around 1.0 g frozen muscle sample was homogenized (1:9, wt/vol) with 154 mmol/L ice-cold sterile sodium chloride solution. The homogenate was centrifuged at 4,450 × g for 15 min at 4°C, and then the supernatant was collected for determination of the contents of total protein, lactate, malondialdehyde (MDA), glutathione (GSH), and the activities of hexokinase (HK), pyruvate kinase (PK), lactate dehydrogenase (LDH), creatine kinase (CK), total superoxide dismutase (T-SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPX), and total antioxidant capacity (T-AOC). All the above assays and muscle glycogen content were quantified using corresponding commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China), as per the manufacturer's instructions.
Messenger RNA Quantification
Total RNA was extracted from the muscle sample using RNAiso reagent (Takara Biotechnology, Dalian, Liaoning, China). Its purity and concentration were quantified by spectrophotometer (ND-1000; Nano Drop Technologies, Wilmington, DE) according to OD260/280 readings (ratio >1.8). Then, RNA sample was diluted in diethyl pyrocarbonate (DEPC) treated water to the same concentration for PCR assays. Reverse transcription and real-time PCR were carried out using the PrimeScript RT Reagent Kit (Takara) and the SYBR Premix Ex Taq II Kit (Takara), respectively, as per the manufacturer's protocols. Real-time PCR was performed on the ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY) as follows: one cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s, and 60°C for 30 s. The sequences of primers for the tested genes were designed and validated using Primer 5.0 software (Table 2), and commercially synthesized by Sangon Biotechnology (Shanghai, China). The 2−ΔΔCT method (Livak and Schmittgen, 2001) was used for the quantification with the glyceraldehyde 3‐phosphate dehydrogena (GAPDH) and β-actin as the internal standard. The mRNA level of each target gene in the control group was assigned a value of one.
Table 2.
Sequences used for real-time PCR primers.
| Genes1 | GeneBank ID | Primer sequence, sense/antisense | Product size (bp) |
|---|---|---|---|
| GYS1 | XM_015275065 | CACGCACCAACAACTTCAAC | 122 |
| CACCAGCAGCGACTCATAGA | |||
| PYGL | NM_204392.1 | CGACTTCAACCTGCGGGACTTC | 192 |
| TTGGGATAGAGGACACGGGAGATG | |||
| GLUT4 | XM_025142445.1 | AAGCACATCCGAACCGCACAC | 220 |
| TCCGTCAGCGAGTCCACATCC | |||
| PRKAG3 | NM_001031258.2 | CCGCCTGCCTGTCATTGAACC | 180 |
| GAGCCGAAGATGTGGAGGAACTTG | |||
| LDHA | NM_205284.1 | CTGTCTGGAGCGGAGTGAATGTTG | 226 |
| GTCCACCACCTGCTTGTGAACC | |||
| LDHB | NM_204177.2 | TTCCGCTACCTGATGGCTGAGAG | 228 |
| TGCCACATTAACTCCGCTCCAAAC | |||
| Nrf2 | NM_205117.1 | GATGTCACCCTGCCCTTAG | 215 |
| CTGCCACCATGTTATTCC | |||
| HO-1 | NM_205344.1 | GCTGGGAAGGAGAGTGAGAGGAC | 214 |
| GCGACTGTGGTGGCGATGAAG | |||
| NQO1 | NM_001277619.1 | CCCGAGTGCTTTGTCTACGAGATG | 214 |
| ATCAGGTCAGCCGCTTCAATCTTC | |||
| CAT | NM_001031215.1 | GGTTCGGTGGGGTTGTCTTT | 211 |
| CACCAGTGGTCAAGGCATCT | |||
| Cu/ZnSOD | NM_205064.1 | CCGGCTTGTCTGATGGAGAT | 124 |
| TGCATCTTTTGGTCCACCGT | |||
| MnSOD | NM_204211.1 | AGGAGGGGAGCCTAAAGGAGA | 214 |
| CCAGCAATGGAATGAGACCTG | |||
| GPX | NM_001277853.1 | GACCAACCCGCAGTACATCA | 205 |
| GAGGTGCGGGCTTTCCTTTA | |||
| γ-GCLc | XM_419910.3 | TGCGGTTCTGCACAAAATGG | 272 |
| TGCTGTGCGATGAATTCCCT | |||
| γ-GCLm | NM_001007953.1 | CCAGAACGTCAAAGCACACG | 187 |
| TCCTCCCATCCCCCAGAAAT | |||
| β-actin | NM_205518.1 | TGCTGTGTTCCCATCTATCG | 150 |
| TTGGTGACAATACCGTGTTCA | |||
| GAPDH | NM_204305.1 | AGAACATCATCCCAGCGTCC | 133 |
| CGGCAGGTCAGGTCAACAAC |
Abbreviations: CAT, catalase; Cu/ZnSOD, copper and zinc superoxide dismutase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GPX, glutathione peroxidase; GYS1, glycogen synthase 1; GLUT4, glucose transporter 4; HO-1, heme oxygenase 1; LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; MnSOD, manganese superoxide dismutase; Nrf2, nuclear factor erythroid 2-related factor 2; NQO1, NAD(P)H quinone dehydrogenase 1; γ-GCLc, γ-glutamate-cysteine ligase catalytic subunit; γ-GCLm, γ-glutamate-cysteine ligase modifier subunit; PRKAG3, protein kinase AMP-activated non-catalytic subunit gamma 3; PYGL, glycogen phosphorylase L.
Statistical Analysis
A cage (replicate) was used as an experimental unit for growth performance and a bird was used as an experimental unit for other parameters. Tukey's multiple range tests of one-way analysis of variance (ANOVA) was used to analyze the differences between any 2 groups. General linear model (GLM) of two-way ANOVA was further used to determine the effect of different sources and levels of betaine. All statistical procedures were performed using SPSS software (Version 20.0 for Windows, SPSS Inc., Chicago, IL), and statistical significance was set at P < 0.05. Results were presented as means and total standard errors of means (SEM).
RESULTS
Growth Performance and Carcass Characteristics
As shown in Table 3, compared with the control group, diet supplemented with anhydrous betaine (L-AB and H-AB groups) significantly improved (P = 0.016) the ADG, and the H-AB group further decreased (P = 0.040) the FCR and increased (P = 0.041) the breast muscle yield of broilers. In addition, broilers fed with anhydrous betaine had higher ADG (P = 0.020) and ADFI (P = 0.003) than those fed with hydrochloride betaine. However, differences in other indicators were not observed among the groups.
Table 3.
Effect of anhydrous betaine and hydrochloride betaine on growth performance and carcass characteristics of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| ADG (g) | 36.6b | 39.1a | 39.1a | 37.9ab | 37.7ab | 0.3 | 0.016 | 0.020 | 0.530 |
| ADFI (g) | 81.4 | 82.9 | 82.7 | 80.5 | 80.4 | 0.4 | 0.052 | 0.003 | 0.750 |
| FCR (g/g) | 2.23a | 2.12ab | 2.11b | 2.13ab | 2.13ab | 0.01 | 0.040 | 0.637 | 0.314 |
| Dressing yield (g/kg) | 900 | 905 | 909 | 906 | 902 | 1 | 0.060 | 0.177 | 0.797 |
| Eviscerated yield (g/kg) | 676 | 692 | 695 | 689 | 688 | 2 | 0.071 | 0.324 | 0.846 |
| Breast muscle yield (g/kg) | 171b | 182ab | 186a | 175ab | 178ab | 2 | 0.041 | 0.052 | 0.342 |
| Thigh muscle yield (g/kg) | 195 | 202 | 207 | 205 | 205 | 2 | 0.200 | 0.899 | 0.523 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
Meat Quality
In breast muscle, the value of pH24h was higher (P = 0.018) in the H-AB and H-HB groups than that in the control group. The value of drip loss24h was lower (P = 0.005) in the L-AB, H-AB, and H-HB groups, and a lower (P = 0.015) value of drip loss48h was also observed in the H-AB group when compared with the control group. Moreover, the anhydrous betaine groups decreased (P = 0.046) the value of drip loss48h compared with the hydrochloride betaine groups. The high-dose betaine groups had a lower (P = 0.047) value of b*45min and a higher (P = 0.047) value of pH24h than the low-dose betaine groups. In thigh muscle, compared with the control group, the value of a*45min was significantly increased (P = 0.037) by H-AB group, and the value of drip loss24h was significantly decreased (P = 0.018) by L-AB and H-AB groups. However, there were no significant differences in the main effects of betaine sources and levels (Table 4).
Table 4.
Effect of anhydrous betaine and hydrochloride betaine on meat quality of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| L*45min | 46.2 | 45.7 | 45.8 | 45.0 | 45.4 | 0.3 | 0.755 | 0.448 | 0.697 |
| a*45min | 6.02 | 6.26 | 6.44 | 6.59 | 5.90 | 0.14 | 0.538 | 0.709 | 0.363 |
| b*45min | 16.6 | 16.8 | 15.9 | 16.8 | 15.1 | 0.3 | 0.319 | 0.539 | 0.047 |
| L*24h | 51.9 | 51.3 | 51.1 | 51.6 | 51.8 | 0.2 | 0.517 | 0.173 | 0.918 |
| a*24h | 7.53 | 8.25 | 8.24 | 7.82 | 7.78 | 0.10 | 0.091 | 0.050 | 0.916 |
| b*24h | 19.6 | 19.9 | 19.9 | 20.1 | 19.6 | 0.1 | 0.796 | 0.940 | 0.463 |
| pH45min | 6.15 | 6.32 | 6.35 | 6.29 | 6.36 | 0.03 | 0.118 | 0.846 | 0.407 |
| pH24h | 5.77b | 5.85ab | 5.98a | 5.90ab | 5.96a | 0.02 | 0.018 | 0.789 | 0.047 |
| Drip loss24h (g/kg) | 39.4a | 30.5b | 26.9b | 32.4ab | 30.3b | 1.1 | 0.005 | 0.191 | 0.170 |
| Drip loss48h (g/kg) | 49.0a | 41.7ab | 39.3b | 45.9ab | 42.9ab | 1.0 | 0.015 | 0.046 | 0.152 |
| Cooking loss (g/kg) | 201 | 185 | 177 | 186 | 183 | 3 | 0.090 | 0.504 | 0.326 |
| Thigh muscle | |||||||||
| L*45min | 46.5 | 44.8 | 45.4 | 45.9 | 45.9 | 0.4 | 0.743 | 0.408 | 0.719 |
| a*45min | 10.1b | 11.7ab | 12.7a | 12.5ab | 11.9ab | 0.3 | 0.037 | 0.994 | 0.727 |
| b*45min | 16.6 | 16.6 | 16.7 | 17.0 | 16.6 | 0.3 | 0.986 | 0.812 | 0.807 |
| L*24h | 50.7 | 48.4 | 50.0 | 49.1 | 49.7 | 0.3 | 0.164 | 0.811 | 0.117 |
| a*24h | 15.6 | 16.5 | 17.2 | 17.2 | 16.9 | 0.2 | 0.211 | 0.758 | 0.775 |
| b*24h | 18.2 | 17.7 | 18.9 | 19.2 | 18.1 | 0.2 | 0.099 | 0.393 | 0.921 |
| pH45min | 6.36 | 6.39 | 6.42 | 6.38 | 6.39 | 0.02 | 0.856 | 0.546 | 0.668 |
| pH24h | 6.08 | 6.13 | 6.17 | 6.13 | 6.13 | 0.01 | 0.422 | 0.512 | 0.512 |
| Drip loss24h (g/kg) | 30.7a | 24.6b | 23.7b | 27.5ab | 25.9ab | 0.7 | 0.018 | 0.124 | 0.450 |
| Drip loss48h (g/kg) | 49.3 | 44.3 | 41.2 | 47.9 | 43.8 | 1.0 | 0.058 | 0.118 | 0.071 |
| Cooking loss (g/kg) | 217 | 193 | 192 | 202 | 197 | 3 | 0.065 | 0.335 | 0.640 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: L*, lightness; a*, redness; b*, yellowness.
Muscle Texture
As indicated in Table 5, the muscle texture of broilers was not significantly affected by dietary betaine supplementation when compared with the control group. However, broilers fed with high-dose betaine had a lower (P = 0.045) cohesiveness of breast muscle than those fed with low-dose betaine.
Table 5.
Effect of anhydrous betaine and hydrochloride betaine on muscle texture of broilers.
| Items | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| Hardness (N) | 1.73 | 1.50 | 1.63 | 1.63 | 1.60 | 0.07 | 0.907 | 0.752 | 0.761 |
| Springness (mm) | 0.917 | 0.851 | 0.951 | 0.870 | 0.839 | 0.018 | 0.242 | 0.289 | 0.430 |
| Cohesiveness | 0.465 | 0.490 | 0.465 | 0.498 | 0.428 | 0.010 | 0.237 | 0.526 | 0.045 |
| Gumminess (N) | 0.819 | 0.729 | 0.767 | 0.774 | 0.692 | 0.037 | 0.861 | 0.850 | 0.777 |
| Chewiness (mJ) | 0.749 | 0.615 | 0.729 | 0.675 | 0.595 | 0.036 | 0.616 | 0.850 | 0.777 |
| Resilience | 0.333 | 0.330 | 0.324 | 0.318 | 0.298 | 0.008 | 0.695 | 0.253 | 0.444 |
| Thigh muscle | |||||||||
| Hardness (N) | 1.71 | 1.09 | 1.34 | 1.41 | 1.43 | 0.09 | 0.259 | 0.253 | 0.462 |
| Springness (mm) | 0.811 | 0.839 | 0.879 | 0.904 | 0.889 | 0.016 | 0.314 | 0.213 | 0.682 |
| Cohesiveness | 0.557 | 0.476 | 0.493 | 0.519 | 0.472 | 0.016 | 0.410 | 0.743 | 0.660 |
| Gumminess (N) | 0.974 | 0.533 | 0.646 | 0.775 | 0.686 | 0.057 | 0.150 | 0.203 | 0.913 |
| Chewiness (mJ) | 0.792 | 0.457 | 0.569 | 0.706 | 0.605 | 0.053 | 0.321 | 0.169 | 0.964 |
| Resilience | 0.414 | 0.324 | 0.318 | 0.358 | 0.312 | 0.017 | 0.313 | 0.705 | 0.479 |
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Muscle Nutrient Composition
Compared with the control group, diet supplemented with high-dose anhydrous betaine (H-AB group) significantly increased (P = 0.040) the content of crude protein in breast muscle. However, no significant differences were observed in the main effects of betaine sources and levels (Table 6).
Table 6.
Effect of anhydrous betaine and hydrochloride betaine on muscle nutrient composition of broilers (fresh basis, g/kg).
| Items | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| Moisture | 732 | 728 | 727 | 731 | 730 | 1 | 0.652 | 0.245 | 0.750 |
| Crude protein | 232b | 240ab | 243a | 237ab | 241ab | 1 | 0.040 | 0.311 | 0.223 |
| Ether extract | 14.6 | 14.0 | 14.8 | 13.5 | 14.3 | 0.5 | 0.931 | 0.669 | 0.503 |
| Thigh muscle | |||||||||
| Moisture | 742 | 738 | 735 | 735 | 735 | 2 | 0.447 | 0.601 | 0.708 |
| Crude protein | 194 | 196 | 196 | 190 | 194 | 1 | 0.286 | 0.086 | 0.444 |
| Ether extract | 44.5 | 52.7 | 49.3 | 48.6 | 51.4 | 0.9 | 0.052 | 0.627 | 0.882 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Muscle NMR Relaxometry
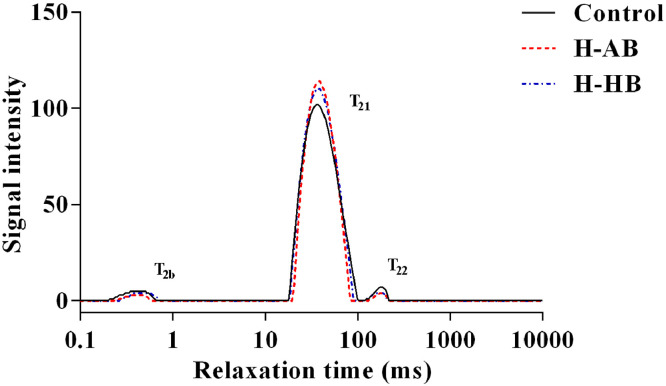
Compared with the control group, H-AB and H-HB groups significantly increased (P = 0.002) immobile water proportion (P21) and decreased (P = 0.030) free water proportion (P22) in breast muscle. In addition, the immobile water relaxation time (T21) in breast muscle was higher (P = 0.011) in the high-dose betaine groups than that in the low-dose betaine group, and the T21 in thigh muscle was higher (P = 0.044) in the hydrochloride betaine groups than that in the anhydrous betaine groups (Table 7). In order to clearly show the water distribution of breast muscle in the groups with significant differences (Control, H-AB, and H-HB groups), the distributed water proton low-field NMR relaxation time curve was presented in Figure 1. Three peaks were detected which represent 3 types of water components in meat: T2b proportions, bound water tightly associated with macromolecules such as proteins; T21 proportions, immobile water; T22 proportions, free water.
Table 7.
Effect of anhydrous betaine and hydrochloride betaine on muscle NMR relaxation times and proportions of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| T2b (ms) | 0.644 | 0.888 | 0.563 | 0.616 | 0.750 | 0.047 | 0.191 | 0.710 | 0.397 |
| T21 (ms) | 84.1 | 84.5 | 75.5 | 82.8 | 76.5 | 1.3 | 0.058 | 0.905 | 0.011 |
| T22 (ms) | 130 | 123 | 121 | 114 | 127 | 4 | 0.670 | 0.779 | 0.292 |
| P2b (%) | 4.13 | 3.79 | 3.45 | 3.78 | 3.81 | 0.11 | 0.422 | 0.484 | 0.539 |
| P21 (%) | 93.5b | 94.5ab | 95.0a | 94.4ab | 94.7a | 0.1 | 0.002 | 0.406 | 0.104 |
| P22 (%) | 2.34a | 1.74ab | 1.54b | 1.78ab | 1.53b | 0.09 | 0.030 | 0.933 | 0.211 |
| Thigh muscle | |||||||||
| T2b (ms) | 0.985 | 1.03 | 0.939 | 0.898 | 0.790 | 0.095 | 0.950 | 0.491 | 0.624 |
| T21 (ms) | 87.6 | 81.7 | 78.4 | 85.6 | 87.3 | 1.6 | 0.309 | 0.044 | 0.787 |
| T22 (ms) | 146 | 158 | 166 | 148 | 152 | 5 | 0.707 | 0.253 | 0.552 |
| P2b (%) | 2.16 | 2.23 | 2.04 | 2.07 | 2.15 | 0.09 | 0.965 | 0.904 | 0.799 |
| P21 (%) | 96.7 | 96.8 | 97.3 | 97.0 | 96.9 | 0.1 | 0.294 | 0.574 | 0.359 |
| P22 (%) | 1.14 | 0.942 | 0.656 | 0.911 | 0.953 | 0.062 | 0.182 | 0.255 | 0.296 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: T2b, bound water relaxation time; T21, immobile water relaxation time; T22, free water relaxation time; P2b, bound water proportion; P21, immobile water proportion; P22, free water proportion.
Figure 1.
Distribution of low-field NMR transverse relaxation times of breast muscle in the control and high-dose betaine groups measured at 12 h postmortem. Control, broilers were fed the basal diet; H-AB, broilers were fed basal diet supplemented with 1,000 mg/kg anhydrous betaine; H-HB, broilers were fed basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine; T2b, bound water relaxation time; T21, immobile water relaxation time; T22, free water relaxation time.
Muscle Postmortem Glycolysis
In breast muscle, compared with the control group, the content of lactate was lower (P = 0.013) in the H-AB group, and the activity of CK was higher (P = 0.007) in the H-AB, L-HB, and H-HB groups. Moreover, broilers fed with high-dose betaine had a lower (P = 0.015) lactate content than those fed with low-dose betaine. However, there were no significant differences in postmortem glycolysis of thigh muscle (Table 8).
Table 8.
Effect of anhydrous betaine and hydrochloride betaine on muscle postmortem glycolysis of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| Glycogen (mg/g) | 3.32 | 3.38 | 3.65 | 3.32 | 3.41 | 0.07 | 0.641 | 0.297 | 0.204 |
| Lactate (mmol/g prot) | 2.84a | 2.56ab | 2.27b | 2.65ab | 2.40ab | 0.06 | 0.013 | 0.283 | 0.015 |
| HK (U/mg prot) | 24.8 | 21.4 | 19.0 | 22.7 | 23.6 | 0.8 | 0.140 | 0.094 | 0.669 |
| PK (U/g prot) | 7.64 | 7.87 | 7.81 | 8.95 | 8.71 | 0.31 | 0.602 | 0.189 | 0.839 |
| LDH (U/g prot) | 223 | 199 | 184 | 189 | 191 | 7 | 0.441 | 0.938 | 0.694 |
| CK (U/mg prot) | 3.06b | 3.72ab | 3.95a | 4.05a | 4.12a | 0.11 | 0.007 | 0.255 | 0.479 |
| Thigh muscle | |||||||||
| Glycogen (mg/g) | 2.41 | 2.34 | 2.57 | 2.25 | 2.35 | 0.05 | 0.353 | 0.145 | 0.114 |
| Lactate (mmol/g prot) | 2.68 | 2.56 | 2.37 | 2.35 | 2.57 | 0.06 | 0.334 | 0.937 | 0.919 |
| HK (U/mg prot) | 11.8 | 10.5 | 9.9 | 10.6 | 9.0 | 0.6 | 0.715 | 0.759 | 0.420 |
| PK (U/g prot) | 19.4 | 17.5 | 16.8 | 17.4 | 17.9 | 0.5 | 0.570 | 0.622 | 0.914 |
| LDH (U/g prot) | 1128 | 1051 | 1021 | 985 | 978 | 18 | 0.055 | 0.151 | 0.627 |
| CK (U/mg prot) | 5.74 | 5.39 | 5.37 | 5.85 | 5.32 | 0.16 | 0.786 | 0.573 | 0.459 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: HK, hexokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; CK, creatine kinase.
Muscle Antioxidant Capacity
Compared with the control group, broilers in the H-AB and H-HB groups had higher GPX activity (P = 0.001) and GSH content (P = 0.015) in breast muscle, and lower MDA contents in breast (P = 0.024) and thigh (P = 0.015) muscles. Moreover, H-HB group further increased (P < 0.05) the activities of T-AOC (P = 0.003) and T-SOD (P = 0.041) in breast muscle and the activity of GPX (P = 0.030) in thigh muscle when compared with the control group. In addition, the activity of GPX (P = 0.008) and the content of GSH (P = 0.022) were higher in the high-dose betaine groups than those in the low-dose betaine groups. However, other indicators did not differ among the treatments (Table 9).
Table 9.
Effect of anhydrous betaine and hydrochloride betaine on muscle antioxidant capacity of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| MDA (nmol/mg prot) | 0.968a | 0.767ab | 0.736b | 0.811ab | 0.744b | 0.026 | 0.024 | 0.408 | 0.131 |
| T-AOC (U/mg prot) | 0.196b | 0.222ab | 0.225ab | 0.224ab | 0.269a | 0.006 | 0.003 | 0.089 | 0.073 |
| T-SOD (U/mg prot) | 22.2b | 24.1ab | 25.2ab | 25.8ab | 28.2a | 0.7 | 0.041 | 0.072 | 0.183 |
| CAT (U/mg prot) | 0.719 | 0.911 | 1.07 | 1.07 | 1.15 | 0.05 | 0.078 | 0.306 | 0.343 |
| GST (U/mg prot) | 30.9 | 30.8 | 33.2 | 33.9 | 32.6 | 1.1 | 0.881 | 0.652 | 0.837 |
| GPX (U/mg prot) | 16.2c | 17.9bc | 20.8ab | 19.4abc | 22.9a | 0.6 | 0.001 | 0.121 | 0.008 |
| GSH (mg/g prot) | 3.78b | 4.04ab | 4.76a | 4.45ab | 4.77a | 0.12 | 0.015 | 0.346 | 0.022 |
| Thigh muscle | |||||||||
| MDA (nmol/mg prot) | 2.30a | 1.81ab | 1.63b | 1.87ab | 1.61b | 0.07 | 0.015 | 0.889 | 0.151 |
| T-AOC (U/mg prot) | 0.373 | 0.416 | 0.384 | 0.388 | 0.456 | 0.012 | 0.212 | 0.442 | 0.509 |
| T-SOD (U/mg prot) | 41.0 | 44.9 | 42.9 | 39.3 | 41.8 | 1.0 | 0.475 | 0.143 | 0.903 |
| CAT (U/mg prot) | 1.95 | 2.12 | 1.97 | 1.70 | 2.01 | 0.07 | 0.512 | 0.305 | 0.685 |
| GST (U/mg prot) | 46.8 | 48.7 | 42.8 | 46.5 | 44.9 | 1.6 | 0.851 | 0.993 | 0.359 |
| GPX (U/mg prot) | 23.1b | 25.5ab | 26.6ab | 27.4ab | 30.0a | 0.7 | 0.030 | 0.103 | 0.245 |
| GSH (mg/g prot) | 8.38 | 8.65 | 8.95 | 7.92 | 8.45 | 0.21 | 0.651 | 0.226 | 0.414 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: CAT, catalase; GST, glutathione S-transferase; GPX, glutathione peroxidase; GSH, glutathione; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase.
Messenger RNA Expression of Glycolytic Genes
As indicated in Table 10, the mRNA expression of glucose transporter 4 (GLUT4) in breast (P = 0.035) and thigh (P = 0.033) muscles, and the mRNA level of protein kinase AMP-activated non-catalytic subunit gamma 3 (PRKAG3) (P = 0.040) in breast muscle were upregulated by H-AB group when compared with the control group. Moreover, the mRNA expression of lactate dehydrogenase B (LDHB) in breast muscle were downregulated (P = 0.036) by high-dose betaine groups compared with low-dose betaine groups. The mRNA levels of glycogen synthase 1 (GYS1) (P = 0.038) and glycogen phosphorylase L (PYGL) (P = 0.020) in thigh muscle were higher in the anhydrous betaine groups than those in the hydrochloride betaine groups. However, the mRNA expressions of other genes tested had no significant differences.
Table 10.
Effect of anhydrous betaine and hydrochloride betaine on mRNA expressions of glycolytic genes in muscle of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| GYS1 | 1.00 | 1.13 | 1.36 | 0.948 | 1.01 | 0.062 | 0.236 | 0.069 | 0.309 |
| PYGL | 1.00 | 1.31 | 1.49 | 1.29 | 1.37 | 0.09 | 0.550 | 0.745 | 0.551 |
| GLUT4 | 1.00b | 1.36ab | 1.69a | 1.17ab | 1.28ab | 0.07 | 0.035 | 0.078 | 0.184 |
| PRKAG3 | 1.00b | 1.89ab | 2.38a | 1.42ab | 1.63ab | 0.15 | 0.040 | 0.080 | 0.307 |
| LDHA | 1.00 | 0.868 | 0.737 | 0.725 | 0.766 | 0.055 | 0.488 | 0.520 | 0.610 |
| LDHB | 1.00 | 0.830 | 0.565 | 0.805 | 0.738 | 0.058 | 0.211 | 0.331 | 0.036 |
| Thigh muscle | |||||||||
| GYS1 | 1.00 | 1.17 | 1.31 | 1.06 | 0.990 | 0.056 | 0.326 | 0.038 | 0.701 |
| PYGL | 1.00 | 1.43 | 1.72 | 1.05 | 0.971 | 0.104 | 0.083 | 0.020 | 0.651 |
| GLUT4 | 1.00b | 1.50ab | 1.72a | 1.33ab | 1.28ab | 0.08 | 0.033 | 0.079 | 0.600 |
| PRKAG3 | 1.00 | 1.29 | 1.18 | 1.40 | 1.38 | 0.29 | 0.914 | 0.678 | 0.856 |
| LDHA | 1.00 | 0.733 | 0.827 | 0.873 | 1.02 | 0.056 | 0.469 | 0.129 | 0.269 |
| LDHB | 1.00 | 0.813 | 1.24 | 0.625 | 0.641 | 0.117 | 0.432 | 0.120 | 0.377 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: GYS1, glycogen synthase 1; GLUT4, glucose transporter 4; LDHA, lactate dehydrogenase A; LDHB, lactate dehydrogenase B; PRKAG3, protein kinase AMP-activated non-catalytic subunit gamma 3; PYGL, glycogen phosphorylase L.
Messenger RNA Expression of Antioxidant Genes
Compared with the control group, the mRNA levels of nuclear factor erythroid 2-related factor 2 (Nrf2) (P = 0.010) and heme oxygenase 1 (HO-1) (P = 0.015) in breast muscle were higher in the H-AB and H-HB groups, and the H-HB group further increased the mRNA expressions of γ-glutamate-cysteine ligase modifier subunit (γ-GCLc) (P = 0.041) in breast muscle and GPX in both breast (P = 0.038) and thigh (P = 0.028) muscles. In addition, the mRNA levels of CAT in breast (P = 0.032) and thigh (P = 0.030) muscles were higher in the anhydrous betaine groups than those in the hydrochloride betaine groups. High-dose betaine supplementation upregulated the mRNA expressions of Nrf2 (P = 0.009) and γ-GCLc (P = 0.044) in breast muscle compared with the low-dose betaine supplementation. But there were no significant differences in the mRNA levels of other antioxidant genes. (Table 11).
Table 11.
Effect of anhydrous betaine and hydrochloride betaine on mRNA expressions of antioxidant genes in muscle of broilers.
| Items4 | Experimental groups1 |
SEM2 |
P-value3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | L-AB | H-AB | L-HB | H-HB | One-way | Source | Level | ||
| Breast muscle | |||||||||
| Nrf2 | 1.00b | 1.33ab | 1.84a | 1.14ab | 1.83a | 0.10 | 0.010 | 0.630 | 0.009 |
| HO-1 | 1.00b | 1.74ab | 1.82a | 1.45ab | 1.81a | 0.09 | 0.015 | 0.470 | 0.284 |
| NQO1 | 1.00 | 1.37 | 1.33 | 0.995 | 1.24 | 0.073 | 0.317 | 0.132 | 0.502 |
| CAT | 1.00 | 1.15 | 1.16 | 0.644 | 0.596 | 0.103 | 0.234 | 0.032 | 0.940 |
| Cu/ZnSOD | 1.00 | 1.27 | 1.17 | 1.06 | 1.16 | 0.06 | 0.645 | 0.439 | 0.994 |
| MnSOD | 1.00 | 1.27 | 0.972 | 1.10 | 1.42 | 0.063 | 0.113 | 0.317 | 0.921 |
| GPX | 1.00b | 1.40ab | 1.48ab | 1.30ab | 1.67a | 0.07 | 0.038 | 0.775 | 0.165 |
| γ-GCLc | 1.00b | 1.34ab | 1.70ab | 1.27ab | 2.00a | 0.11 | 0.041 | 0.655 | 0.044 |
| γ-GCLm | 1.00 | 1.22 | 1.19 | 1.12 | 1.22 | 0.05 | 0.656 | 0.777 | 0.786 |
| Thigh muscle | |||||||||
| Nrf2 | 1.00 | 1.15 | 1.50 | 1.22 | 1.45 | 0.08 | 0.211 | 0.967 | 0.120 |
| HO-1 | 1.00 | 1.61 | 1.21 | 1.07 | 1.56 | 0.14 | 0.552 | 0.792 | 0.902 |
| NQO1 | 1.00 | 1.24 | 1.19 | 1.70 | 1.09 | 0.09 | 0.129 | 0.405 | 0.132 |
| CAT | 1.00 | 1.33 | 1.32 | 1.10 | 0.727 | 0.094 | 0.227 | 0.030 | 0.291 |
| Cu/ZnSOD | 1.00 | 1.08 | 1.30 | 1.10 | 1.05 | 0.06 | 0.581 | 0.435 | 0.569 |
| MnSOD | 1.00 | 0.995 | 1.19 | 0.893 | 1.11 | 0.060 | 0.595 | 0.544 | 0.159 |
| GPX | 1.00b | 1.40ab | 1.89ab | 1.91ab | 2.00a | 0.12 | 0.028 | 0.262 | 0.284 |
| γ-GCLc | 1.00 | 1.57 | 1.28 | 1.23 | 1.30 | 0.09 | 0.416 | 0.452 | 0.613 |
| γ-GCLm | 1.00 | 1.04 | 1.13 | 0.926 | 0.972 | 0.047 | 0.732 | 0.210 | 0.532 |
Means within a row with different superscripts differ significantly at P < 0.05.
Control, broilers were fed the basal diet; L-AB, broilers were fed the basal diet supplemented with 500 mg/kg anhydrous betaine; H-AB, broilers were fed the basal diet supplemented with 1,000 mg/kg anhydrous betaine; L-HB, broilers were fed the basal diet supplemented with 642.23 mg/kg hydrochloride betaine; H-HB, broilers were fed the basal diet supplemented with 1,284.46 mg/kg hydrochloride betaine.
SEM, standard error of means (n = 8).
One-way, differences among 5 treatments (one-way analysis of variance); Source and Level, differences of betaine sources (L-AB + H-AB × L-HB + H-HB) and levels (L-AB + L-HB × H-AB + H-HB), respectively (two-way analysis of variance).
Abbreviations: CAT, catalase; Cu/ZnSOD, copper and zinc superoxide dismutase; GPX, glutathione peroxidase; HO-1, heme oxygenase 1; MnSOD, manganese superoxide dismutase; Nrf2, nuclear factor erythroid 2-related factor 2; NQO1, NAD(P)H quinone dehydrogenase 1; γ-GCLc, γ-glutamate-cysteine ligase catalytic subunit; γ-GCLm, γ-glutamate-cysteine ligase modifier subunit.
DISCUSSION
As a stable and nontoxic feed additive, betaine has been widely used in animal diets due to its lipotropic and growth-promoting effects (Eklund et al., 2005). In this study, diet supplemented with anhydrous betaine significantly improved the ADG of broilers, and the H-AB group further decreased the FCR and increased the breast muscle yield when compared with the control group. Similar result was obtained by Song et al. (2021) who reported that diet supplemented with 1,000 mg/kg anhydrous betaine significantly increased the ADG and decreased FCR of yellow-feathered broilers. Some studies had also shown the positive effects of betaine on growth performance and carcass characteristics in broilers and pigs (Zhan et al., 2006, Wang et al., 2020; Wen et al., 2021). This might be due to the fact that betaine could regulate myogenic genes expression and the insulin-like growth factor-1 signaling pathway to increase muscle protein deposition (Zhan et al., 2006, Chen et al., 2018). In addition, the present study demonstrated that broilers fed anhydrous betaine had higher ADG and ADFI than those fed hydrochloride betaine. Anhydrous betaine tastes sweet, while hydrochloride betaine tastes bitter, which may affect the feed palatability and the feed intake of broilers. Moreover, the process of nutrient digestion and absorption is dependent on an intact gut epithelium, so the osmotic capacity of betaine may positively affect digestibility (Eklund et al., 2005; Metzler et al., 2009). Anhydrous betaine shows better osmotic capacity than hydrochloride betaine due to its higher solubility. Therefore, broilers fed with anhydrous betaine may have better digestibility than those fed with hydrochloride betaine, which also explains why the ADG and ADFI were higher in the anhydrous betaine groups than those in the hydrochloride betaine groups.
Meat quality is important for producers and, above all, consumers. In addition to sensory attributes (color, juiciness, and flavor), the quality of meat is reflected in its physicochemical parameters, such as WHC, texture, and nutrient composition (Duclos et al., 2007; Grashorn, 2010). Previous studies had confirmed that betaine had positive effects on meat quality of farm animals (Matthews et al., 2001; Attia et al., 2009; Wen et al., 2019; Dong et al., 2020). In this study, diet supplemented with anhydrous betaine significantly decreased drip loss24h in breast and thigh muscles, and high-dose anhydrous betaine further increased pH24h and decreased drip loss48h in breast muscle. Moreover, the H-HB group also significantly increased pH24h and decreased drip loss24h in breast muscle. It is believed that high ultimate pH is closely related to decreased drip loss, which leads to great WHC of meat (Duclos et al., 2007). So, our results implied that betaine could enhance muscle WHC of broilers by increasing ultimate pH and decreasing drip loss. Similar results were reported in broilers, pigs, and lambs, that dietary betaine supplementation could decrease muscle drip loss (Matthews et al., 2001; Chen et al., 2020; Dong et al., 2020). Increased water retention of muscle tissue may be attributed to the osmotic capacity of betaine. Anhydrous betaine shows higher solubility in water than hydrochloride betaine, thereby increasing its osmotic capacity. This might be the reason why broilers fed with anhydrous betaine had lower drip loss48h in breast muscle than those fed with hydrochloride betaine in this study. Lactic acid accumulation is the main reason for the muscle pH decrease after slaughter. The higher breast muscle pH24h by high-dose betaine supplementation in this study suggested that betaine could affect muscle postmortem glycolysis to mitigate lactate accumulation and protein denaturation, which in turn decreased the drop loss of meat (Matthews et al., 2001). In addition, the value of a*45min in thigh muscle was increased by high-dose anhydrous betaine supplementation, which might be attributed to an increased myoglobin content in muscle by dietary betaine, ultimately deepening the meat color (Brewer, 2004; Yu et al., 2004).
Muscle texture, including hardness, springness, cohesiveness, gumminess, chewiness, and resilience, is an important physical property of meat, which can be used to evaluate meat quality digitally by simulating oral chewing (Paris and Mourtzakis, 2021). In the present study, the texture characteristics of meat were not significantly affected by dietary betaine supplementation when compared with the control group. However, diet supplemented with high-dose anhydrous betaine significantly increased crude protein content in breast muscle, and high-dose hydrochloride betaine also numerically increased breast muscle crude protein content by 3.79%. Nutrient composition is a vital meat quality feature that regards to human nutrition (Grashorn, 2010). An increased crude protein content in breast muscle meant that the edible value of meat was improved by betaine supplementation. This effect may be due to the fact that betaine can take part in methionine (Met) cycle to spare Met by acting as a methyl donor, thus more Met can be used for muscle protein synthesis (Eklund et al., 2005; Corzo et al., 2006). Low-field NMR relaxation measurement is regarded as a useful method to estimate muscle WHC, because it gives a direct measure of the proportion of water in the fresh meat (Bertram et al., 2002). As shown in Figure 1, three relation proportions (T2b, T21, and T22) represented 3 states of water in meat: bound water, immobilized water, and free water, respectively, in which free water is most likely to be lost after slaughter. The data in this study demonstrated that dietary high-dose betaine supplementation significantly increased P21 proportion and decreased P22 proportion in breast muscle, indicating that betaine could affect water distribution of fresh meat to enhance muscle WHC. This result was in accordance with the lower drop loss of breast muscle in the H-AB and H-HB groups. There was evidence that betaine, as an osmolyte, could protect the cell against dehydration and increase water retention, thereby resulting in higher immobilized water and lower free water proportions in breast muscle (Attia et al., 2005, 2009; Hoffman et al., 2009).
Postmortem metabolism of muscle tissue, especially the rate of acidification, has strong effects on meat quality (Duclos et al., 2007). During the initiation of rigor mortis, muscle metabolism is modified due to the cessation of oxygen supply. Then, muscle relies on the anaerobic glycolysis pathway to use the glycogen stores for ATP regeneration, which leads to lactic acid accumulation (Duclos et al., 2007; Stephens et al., 2008). In this study, compared with the control group, diet supplemented with high-dose anhydrous betaine significantly decreased lactate content in breast muscle, and high-dose hydrochloride betaine also numerically decreased breast muscle lactate content by 15.49%, which was consistent with the result of higher pH24h value in breast muscle in the H-AB and H-HB groups. This finding was similar to the data of Warren et al. (1999), who reported that betaine supplementation could decrease muscle lactate accumulation of horses. Our results also showed that muscle anaerobic glycolysis was not affected by betaine source, but high-dose betaine groups had lower lactate content in breast muscle than low-dose betaine groups, indicating that the accumulation rate of muscle lactate was dose-dependent in betaine treatments. It is well known that the rate of anaerobic glycolysis is mediated by some key enzymes, such as HK, PK, and LDH, which convert glucose to glucose-6-phosphate, phosphoenolpyruvate to pyruvate, and pyruvate to lactic acid, respectively (Scheffler and Gerrard, 2007; Nelson and Cox, 2008). In this study, the activities of HK, PK, and LDH in muscle were not affected by dietary betaine supplementation, which revealed that betaine might have no significant effects on the muscle anaerobic glycolysis rate of broilers under normal environment. However, the activity of CK was higher in the H-AB, L-HB, and H-HB groups. It has been reported that betaine can increase the content of creatine in muscle, which can be further converted into phosphocreatine by CK (Craig, 2004). As the main source of energy stored in muscle, phosphocreatine can be used for contracting muscle fibers and anaerobic effort (Fitch and Shields, 1966; Anna and Greenhaff, 2000). A higher CK activity in breast muscle by betaine supplementation indicated that the rate of creatine conversion to phosphocreatine was enhanced, and more energy might be stored in muscle. Therefore, muscle postmortem metabolism needed less energy provided by glycolytic pathway, and the production of lactate was correspondingly reduced in the high-dose betaine group. But this effect was not found in thigh muscle, which might be due to the different fiber types of breast and thigh muscles. GLUT4 is the only transporter of blood glucose, an essential energy source, into skeletal muscle, and PRKAG3 can promote muscle absorption of glucose and regulate cellular energy homeostasis by activating 5’-adenosine-monophosphate-activated protein kinase (AMPK) (Huang and Czech, 2007; Pinter et al., 2013; Yang et al., 2016). Thus, these 2 genes play a key role in regulating muscle energy metabolism. In the present study, the mRNA expression of GLUT4 in breast and thigh muscles, and the mRNA level of PRKAG3 in breast muscle were upregulated by high-dose anhydrous betaine supplementation, which revealed that anhydrous betaine could improve muscle energy metabolism. Considering that the protein deposition is closely related to muscle energy metabolism, higher breast muscle protein content in high-dose anhydrous betaine group may be due to improved muscle energy metabolism (Li et al., 2020). In addition, studies found that PRKAG3 gene was positively correlated with meat quality by decreasing drip loss (Ciobanu et al., 2001; Yang et al., 2016). Thus, betaine might improve muscle WHC of broilers by regulating PRKAG3 gene expression.
Meat oxidation, especially lipid peroxidation, is one of the most important reasons for meat quality deterioration, which reduces meat nutritive value and causes texture problems (Salih et al., 1987; Fernandez et al., 1997). MDA, a metabolite derived from lipid peroxidation, has been widely used as an indicator of oxidative damage (Alirezaei et al., 2012). In this study, diet supplemented with high-dose betaine significantly decreased the content of MDA in breast and thigh muscles, indicating that betaine could alleviate oxidative damage. Enzymatic and non-enzymatic antioxidant systems are body's main antioxidant defense systems, which play an important role in maintaining redox balance (Michiels et al., 1994). SOD is a key antioxidant enzyme that can convert the superoxide anion (O2•−) into less dangerous hydrogen peroxide (H2O2) (Alirezaei et al., 2012). CAT, GST, and GPX, other antioxidant enzymes in cell, can then decompose H2O2 to water (Kheradmand et al., 2010). GSH is one of the non-enzymatic antioxidants which scavenge free radicals under the GSH antioxidant system, and T-AOC, an integrative index, can reflect the body's total antioxidant level (Wu et al., 2004; Deng et al., 2018). Therefore, T-AOC, SOD, CAT, GST, GPX, and GSH are often used as effective indicators to reflect the antioxidant capacity of animals. Our study demonstrated that dietary high-dose betaine supplementation significantly increased the activity of GPX and the content of GSH in breast muscle, and H-HB group further increased the activities of T-AOC and T-SOD in breast muscle, and thigh muscle GPX activity when compared with the control group. However, there were no significant differences between these two sources of betaine. The improved antioxidant enzymes activities and GSH content combined with reduced MDA content in muscle revealed that dietary betaine could improve muscle antioxidant capacity. Similar results were reported by Alirezaei et al. (2012), who found that diet supplemented with 1,000 mg/kg betaine could decrease lipid peroxidation and improve antioxidant enzymes activities in breast muscle of broilers. The positive effects of dietary betaine on muscle antioxidant capacity were also demonstrated in ducks, lambs, and fish (Adjoumani et al., 2017; Chen et al., 2019; Dong et al., 2020). To investigate the molecular mechanism by which muscle antioxidant capacity was regulated by betaine, the mRNA expressions of antioxidant genes were measured in the present study. The Nrf2 is an important transcription factor that plays a fundamental role in cellular defense against reactive free radicals and other oxidant species (Silva-Islas et al., 2019). HO-1, a downstream antioxidant gene of Nrf2 signaling pathway, is involved in the oxidative degradation of heme (Loboda et al., 2016). Our data showed that the mRNA expressions of Nrf2 and HO-1 in breast muscle were upregulated by high-dose betaine supplementation, which indicated that betaine might improve muscle antioxidant capacity by altering Nrf2 signaling pathway. In addition, the mRNA expressions of GPX and γ-GCLc in breast muscle, and GPX in thigh muscle were higher in the H-HB group than those in the control group. It had been reported that betaine could participate in the methionine cycle to increase the supply of substrates required for the synthesis of GSH, where γ-GCLc played a vital role in catalyzing the condensation of cysteine and glutamate to form the GSH (Craig, 2004; Farina and Aschner, 2019). Therefore, a higher GSH content in the high-dose betaine group might be due to the increased mRNA expression of γ-GCLc. As a low-molar sulfhydryl antioxidant, GSH can directly react with hydroxyl (OH•) and O2•−, or donate electrons for the reduction of H2O2 and organic peroxides in a reaction catalyzed by enzymes of GPX family (Farina and Aschner, 2019). So, the GSH and the main enzymes involved in its metabolism together form the GSH antioxidant system. Considering the higher GSH content and the activity as well as mRNA expression of GPX in the high-dose betaine group, our study demonstrated that the antioxidant effect of betaine was significantly favored by GSH antioxidant system.
CONCLUSIONS
In summary, anhydrous betaine showed better effects than hydrochloride betaine in improving growth performance and breast muscle yield of partridge shank broiler chickens. Moreover, anhydrous betaine (1,000 mg/kg) or equimolar hydrochloride betaine supplementation could improve the meat quality of broilers by reducing lactate content to increase muscle ultimate pH, influencing meat water distribution (increasing immobile water proportion and decreasing free water proportion) to decrease drip loss, and enhancing muscle antioxidant capacity. Considering both growth performance and meat quality, 1,000 mg/kg anhydrous betaine is recommended for broilers.
ACKNOWLEDGMENTS
This study was funded by grants from Cooperative Innovation Foundation of Industry-Prospective Joint Research Projects of Jiangsu Province (BY2014128-03)
DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- Adjoumani J.Y., Wang K.Z., Zhou M., Liu W.B., Zhang D.D. Effect of dietary betaine on growth performance, antioxidant capacity and lipid metabolism in blunt snout bream fed a high-fat diet. Fish Physiol. Biochem. 2017;43:1733–1745. doi: 10.1007/s10695-017-0405-9. [DOI] [PubMed] [Google Scholar]
- Alirezaei M., Gheisari H.R., Ranjbar V.R., Hajibemani A. Betaine: a promising antioxidant agent for enhancement of broiler meat quality. Br. Poult. Sci. 2012;53:699–707. doi: 10.1080/00071668.2012.728283. [DOI] [PubMed] [Google Scholar]
- Anna C., Greenhaff P.L. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am. J. Clin. Nutr. 2000;72:607S–617S. doi: 10.1093/ajcn/72.2.607S. [DOI] [PubMed] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists; Gaithersburg, MD: 2000. Official Methods of Analysis. [Google Scholar]
- Arshad M.S., Anjum F.M., Asghar A., Khan M.I., Yasin M., Shahid M., El-Ghorab A.H. Lipid stability and antioxidant profile of microsomal fraction of broiler meat enriched with α-lipoic acid and α-tocopherol acetate. J. Agr. Food Chem. 2011;59:7346–7352. doi: 10.1021/jf2002393. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Abd El-Hamid A.E., Abdallah A.A., Berikaa M.A., El-Gandy M.F., Sahin K., Abou-Shehema B.M. Effect of betaine, vitamin C and vitamin E on egg quality, hatchability, and markers of liver and renal functions in dual-purpose breeding hens exposed to chronic heat stress. Eur. Poult. Sci. 2018;82:1612–9199. [Google Scholar]
- Attia Y.A., El-Hamid E.A., Abedalla A., Berika M.A., Al-Harthi M.A., Kucuk O., Sahin K., Abou-Shehema B.M. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. Springerplus. 2016;5:1619. doi: 10.1186/s40064-016-3304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Hassan R.A., Qota E.M.A. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1: effect of ascorbic acid and different levels of betaine. Trop. Anim. Health Prod. 2009;41:807–818. doi: 10.1007/s11250-008-9256-9. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Hassan R.A., Shehatta M.H., Abd El-Hady S.B. Growth, carcass quality and serum constituents of slow growing chicks as affected by betaine addition to diets containing 2. Different levels of methionine. Int. J. Poult. Sci. 2005;4:856–865. [Google Scholar]
- Bertram H.C., Purslow P.P., Andersen H.J. Relationship between meat structure, water mobility, and distribution: a low-field nuclear magnetic resonance study. J. Agr. Food. Chem. 2002;50:824–829. doi: 10.1021/jf010738f. [DOI] [PubMed] [Google Scholar]
- Brewer S. Irradiation effects on meat color - a review. Meat Sci. 2004;68:1–17. doi: 10.1016/j.meatsci.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Chen R., Wen C., Gu Y.F., Wang C., Chen Y.P., Zhuang S., Zhou Y.M. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food Agric. 2020;100:2656–2663. doi: 10.1002/jsfa.10296. [DOI] [PubMed] [Google Scholar]
- Chen R., Wen C., Cheng Y.F., Chen Y.P., Zhuang S., Zhou Y.M. Effects of dietary supplementation with betaine on muscle growth, muscle amino acid contents and meat quality in Cherry Valley ducks. J. Anim. Physiol. Anim. Nutr. 2019;103:1050–1059. doi: 10.1111/jpn.13083. [DOI] [PubMed] [Google Scholar]
- Chen R., Zhuang S., Chen Y.P., Cheng Y.F., Wen C., Zhou Y.M. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018;97:4297–4305. doi: 10.3382/ps/pey303. [DOI] [PubMed] [Google Scholar]
- Ciobanu D., Bastiaansen J., Malek M., Helm J., Woollard J., Plastow G., Rothschild M. Evidence for new alleles in the protein kinase adenosine monophosphate-activated γ3-Subunit gene associated with low glycogen content in pig skeletal muscle and improved meat quality. Genetics. 2001;159:1151–1162. doi: 10.1093/genetics/159.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo A., Kidd M.T., Dozier W.A., Shack L.A., Burgess S.C. Protein expression of pectoralis major muscle in chickens in response to dietary methionine status. Br. J. Nutr. 2006;95:703–708. doi: 10.1079/bjn20051716. [DOI] [PubMed] [Google Scholar]
- Craig S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- Deng K.P., Fan Y.X., Ma T.W., Wang Z., Tantai W.J., Nie H.T., Guo Y.X., Yu X.Q., Su L.W., Wang F. Carcass traits, meat quality, antioxidant status and antioxidant gene expression in muscle and liver of Hu lambs fed perilla seed. J. Anim. Physiol. Anim. Nutr. 2018;102:828–837. doi: 10.1111/jpn.12841. [DOI] [PubMed] [Google Scholar]
- Dong L., Zhong Z.X., Cui H.H., Wang S.N., Luo Y., Yu L.H., Loor J.J., Wang H.R. Effects of rumen-protected betaine supplementation on meat quality and the composition of fatty and amino acids in growing lambs. Animal. 2020;14:435–444. doi: 10.1017/S1751731119002258. [DOI] [PubMed] [Google Scholar]
- Duclos M.J., Berri C., Bihan-Duval E.L. Muscle growth and meat quality. J. Appl. Poult. Res. 2007;16:107–112. [Google Scholar]
- Eklund M., Bauer E., Wamatu J., Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- Farina M., Aschner M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim. Biophys. Acta-Gen. Subj. 2019;1863 doi: 10.1016/j.bbagen.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J., Perez-Alvarez J.A., Fernandez-Lopez J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59:345–353. [Google Scholar]
- Fitch C.D., Shields R.P. Creatine metabolism in skeletal muscle. I. Creatine movement across muscle membranes. J. Bioll. Chem. 1966;241:3611–3614. [PubMed] [Google Scholar]
- Grashorn M.A. Research into poultry meat quality. Br. Poult. Sci. 2010;51:60–67. doi: 10.1080/00071668.2010.506761. [DOI] [PubMed] [Google Scholar]
- Hoffman J.R., Ratamess N.A., Kang J., Rashti S.L., Faigenbaum A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sport Nutr. 2009;6:7. doi: 10.1186/1550-2783-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Kheradmand A., Alirezaei M., Birjandi M. Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept. 2010;162:84–89. doi: 10.1016/j.regpep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Li L.Y., Li J.M., Ning L.J., Lu D.L., Luo Y., Ma Q., Limbu S.M., Li D., Chen L., Lodhi I.J., Degrace P., Zhang M.L., Du Z.Y. Mitochondrial fatty acid β-oxidation inhibition promotes glucose utilization and protein deposition through energy homeostasis remodeling in fish. J. Nutr. 2020;150:2322–2335. doi: 10.1093/jn/nxaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.O., Southern L.L., Bidner T.D., Persica M.A. Effects of betaine, pen space, and slaughter handling method on growth performance, carcass traits, and pork quality of finishing barrows. J. Anim. Sci. 2001;79:967–974. doi: 10.2527/2001.794967x. [DOI] [PubMed] [Google Scholar]
- Metzler B.U., Eklund M., Mosenthin R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. Worlds Poult. Sci. J. 2009;65:419–441. [Google Scholar]
- Michiels C., Raes M., Toussaint O., Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994;17:235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Nelson, D. L., and M. M. Cox. 2008. Glycolysis, gluconeogenesis, and the pentose phosphate pathway. Pages 527-568 in Lehninger Principles of Biochemistry. W. H. Freeman and Company, New York, NY.
- Paris M.T., Mourtzakis M. Muscle composition analysis of ultrasound images: a narrative review of texture analysis. Ultrasound Med. Biol. 2021;47:880–895. doi: 10.1016/j.ultrasmedbio.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Petracci M., Bianchi M., Cavani C. The European perspective on pale, soft, exudative conditions in poultry. Poult. Sci. 2009;88:1518–1523. doi: 10.3382/ps.2008-00508. [DOI] [PubMed] [Google Scholar]
- Pinter K., Grignani R.T., Watkins H., Redwood C. Localisation of AMPK γ subunits in cardiac and skeletal muscles. J. Muscle Res. Cell Motil. 2013;34:369–378. doi: 10.1007/s10974-013-9359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih A.M., Smith D.M., Price J.F., Dawson L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987;66:1483–1488. doi: 10.3382/ps.0661483. [DOI] [PubMed] [Google Scholar]
- Scheffler T.L., Gerrard D.E. Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci. 2007;77:7–16. doi: 10.1016/j.meatsci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Silva-Islas C.A., Chanez-Cardenas M.E., Barrera-Oviedo D., Ortiz-Plata A., Pedraza-Chaverri J., Maldonado P.D. Diallyl trisulfide protects rat brain tissue against the damage induced by ischemia-reperfusion through the Nrf2 pathway. Antioxidants. 2019;8:410. doi: 10.3390/antiox8090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.D., Chen R., Yang M., Liu Q., Zhou Y.M., Zhuang S. Dietary betaine supplementation improves growth performance, digestive function, intestinal integrity, immunity, and antioxidant capacity of yellow-feathered broilers. Ital. J. Anim. Sci. 2021;20:1575–1586. [Google Scholar]
- Stephens J.W., Dikeman M.E., Unruh J.A., Haub M.D., Tokach M.D., Dritz S.S. Effects of oral administration of sodium citrate or acetate to pigs on blood parameters, postmortem glycolysis, muscle pH decline, and quality attributes of pork. J. Anim. Sci. 2008;86:1669–1677. doi: 10.2527/jas.2007-0797. [DOI] [PubMed] [Google Scholar]
- Wang T., Li J., Shao Y., Yao W., Xia J., He Q., Huang F. The effect of dietary garcinol supplementation on oxidative stability, muscle postmortem glycolysis and meat quality in pigs. Meat Sci. 2020;161 doi: 10.1016/j.meatsci.2019.107998. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Chen X., Tan H.Z., Zhang D.X., Zhang H.J., Wei S., Yan H.C. Nutrient density and slaughter age have differential effects on carcase performance, muscle and meat quality in fast and slow growing broiler genotypes. Br. Poult. Sci. 2013;54:50–61. doi: 10.1080/00071668.2012.745927. [DOI] [PubMed] [Google Scholar]
- Warren L.K., Lawrence L.M., Thompson K.N. The influence of betaine on untrained and trained horses exercising to fatigue. J. Anim. Sci. 1999;77:677–684. doi: 10.2527/1999.773677x. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen Y.P., Leng Z.Y., Ding L.R., Wang T., Zhou Y.M. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agric. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- Wen C., Chen R., Chen Y.P., Ding L.R., Wang T., Zhou Y.M. Betaine improves growth performance, liver health, antioxidant status, breast meat yield, and quality in broilers fed a mold-contaminated corn-based diet. Anim. Nutr. 2021;7:661–666. doi: 10.1016/j.aninu.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yang Y., Dan X., Ling Y., Zhao C. An SNP in exon 11 of chicken 5′-AMP-activated protein kinase gamma 3 subunit gene was associated with meat water holding capacity. Anim. Biotechnol. 2016;27:13–16. doi: 10.1080/10495398.2015.1069300. [DOI] [PubMed] [Google Scholar]
- Yu D.Y., Xu Z.R., Li W.F. Effects of betaine on growth performance and carcass characteristics in growing pigs. Asian-Australas. J. Anim. Sci. 2004;17:1700–1704. [Google Scholar]
- Zhan X. A., J. X. Li, Z. R. Xu, and R. Q. Zhao. 2006. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 47:576–580 [DOI] [PubMed]
- Zhang L., Wang X., Li J., Zhu X., Gao F., Zhou G.H. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J. Agr. Food Chem. 2017;65:6991–6999. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]