Abstract
Purpose
Studies on the immune responses to severe acute respiratory syndrome coronavirus 2 vaccines are necessary to evaluate the ongoing vaccination programs by correlating serological response data and clinical effectiveness data. We performed a longitudinal immunological profiling of health care workers vaccinated with mRNA-1273 (Moderna, Cambridge, MA, USA). Half of these vaccinees had experienced a mild coronavirus disease 2019 (COVID-19) infection in the spring of 2020 (“COVID-recovered” cohort), whereas the other half of the vaccinees had no previous COVID-19 infection (“COVID-naive” cohort).
Materials and Methods
Serum was drawn at multiple time points and subjected to assays measuring anti-Spike immunoglobulin G (IgG), avidity of anti-Spike IgG, avidity of anti-receptor binding domain (RBD) IgG, virus neutralizing activity, and interferon-γ release from stimulated lymphocytes.
Results
Between both cohorts and within each cohort, we found remarkable inter-individual differences regarding cellular and humoral immune responses to the Moderna mRNA-1273 vaccine.
Conclusion
First, our study indicates that the success of mRNA-1273 vaccinations should be verified by serological assays in order to identify “low-responders” to vaccination. Second, the kinetics of anti-S IgG and neutralizing activity correlate well with clinical effectiveness data, thus explaining incipient protection against infection 2 weeks after the first dose of mRNA-1273 in COVID-naive vaccinees. Third, our IgG-avidity data indicate that this incipient protection is mediated by low-avidity anti-RBD IgG and low-avidity anti-S IgG.
Keywords: COVID-19, Non-responders, Vaccination, Avidity, SARS-CoV-2
Introduction
The new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 causing a pandemic [1,2]. Ongoing vaccination efforts are aimed at mitigating it. Studies on the immune responses to SARS-CoV-2 vaccines are necessary to evaluate these vaccination programs by correlating serological response data and clinical effectiveness data. Non-responders and low-responders to vaccinations are often encountered in clinical medicine (e.g., regarding hepatitis B) [3]. We wished to confirm serological responses to SARS-CoV-2 vaccination for the health care workers (HCWs) in our department which was performed with two doses of mRNA-1273 (Moderna, Cambridge, MA, USA) applied within 4 weeks.
Materials and Methods
Serological assays
The Liaison “SARS-CoV-2 S1/S2 IgG” assay from DiaSorin (Saluggia, Italy), the surrogate virus neutralization test (sVNT) “cPass Neutralization Antibody Detection Kit” from GenScript (Piscataway, NJ, USA), and the “recomLine SARS-Cov-2 IgG avidity” assay from Mikrogen (Neuried, Germany) were performed according the manufacturer’s instructions (cut-off for DiaSorin >15 AU/mL; cut-off for Mikrogen >60%). For the GenScript assay, a cut-off of 30% inhibition was applied according to Meyer et al. [4] who recently validated this novel test. The QuantiFERON SARS-CoV-2 Interferon-γ release assay (Qiagen, Hilden, Germany) was also performed according to the manufacturer’s recommendations.
Study design
Six HCWs volunteered to donate serum at multiple time points. Half of the volunteers had experienced a mild laboratory-confirmed coronavirus disease 2019 (COVID-19) infection in the spring of 2020 (the “COVID-recovered” vaccinee cohort), the other half of the volunteers were without previous COVID-19 infection (the “COVID-naive” vaccine cohort). The median age in the COVID-naive group (denoted a, b, c) was 56 years, the median age in the COVID-recovered group (denoted d, e, f) was 61 years. The female:male ratio in both groups was 1:2. Interestingly, one COVID-recovered vaccinee ‘f’ received only one vaccination at day 1 and no second dose due to general vaccine shortage and a change in vaccination recommendations during the course of this study.
Vaccinations were performed at day 1 and 28, time point “0” refers to control sera taken before the first vaccination. Some participants could unfortunately not provide samples at each planned time point: vaccinee ‘d’ at 3 time points: day 6–7, day 31–32, and day 37–40; vaccinee ‘f’ at 2 time points: day 34–35 and day 37–40; and vaccinee ‘b’ at 1 time point: day 51–54. Samples for the interferon-γ (INF-γ) release assay were obtained 12–13 weeks after the first vaccination.
Ethics statement
Written informed consent of all volunteers was obtained. Sample and data acquisition were approved by the Medical Ethics Committee of the University Hospital RWTH Aachen (EK 093/20).
Results
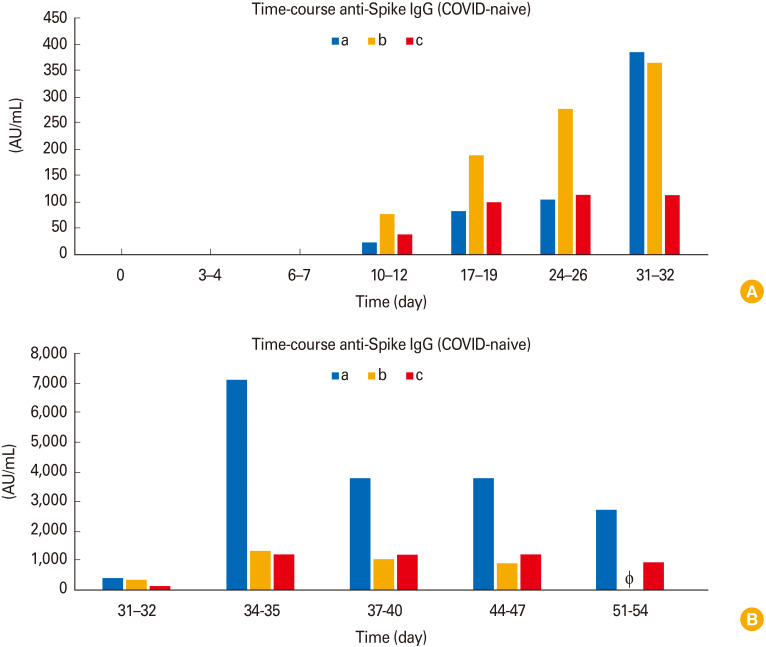
Using the DiaSorin anti-S1/S2 IgG assay (a chemiluminescence immunoassay), we found that COVID-naive vaccinees did not show anti-S immunoglobulin G (IgG) before vaccination (day 0) and at day 3–4 and day 6–7 after their first vaccination [5] (Fig. 1A). Seroconversion was first observed at day 10–12 after first vaccination when anti-S IgG became measurable (Fig. 1A depicts day 0–32 and Fig. 1B depicts day 32–54). Peak levels of anti-S IgG were reached around day 34–35 (7 days after the second vaccination performed at day 28). Interestingly, we noticed remarkable quantitative inter-individual differences between single vaccinees: for example, at time point 34–35 days (the time point of peak levels), vaccinee ‘a’ showed high levels of anti-S IgG (7,140 AU/mL) whereas vaccinee ‘c’ showed much lower levels (1,200 AU/mL). This amounts to an almost sixfold difference between these two individuals vaccinated with mRNA-1273.
Fig. 1. (A, B) Time course of anti-Spike immunoglobulin G (IgG) in coronavirus disease (COVID)-naive vaccinees (individuals a, b, and c). Vaccinations were performed at day 1 and 28. One participant (c) could not provide a sample at day 51–54. The lacking sample is depicted as Φ.
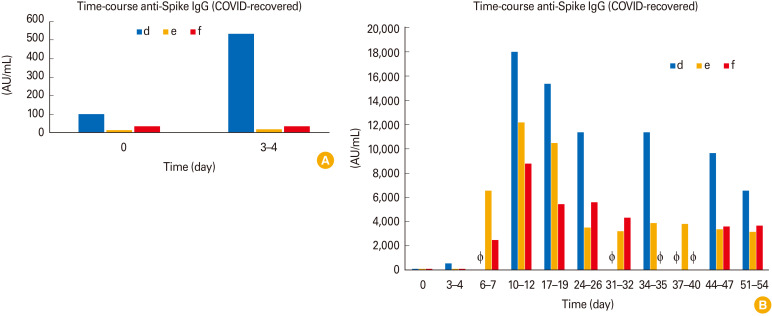
After profiling our COVID-naive cohort, we next examined the serological profile of our second cohort, the COVID-recovered vaccinees (Fig. 2). As perhaps expected, all COVID-recovered vaccinees already had low levels of anti-S IgG before their first vaccination (Fig. 2A depicts day 0 and 3–4, whereas Fig. 2B depicts the full time-course). Subsequently, anti-S IgG rapidly increased to reach peak levels during the second week after the first dose in all COVID-recovered vaccinees (day 10–12). Surprisingly, the second vaccine dose at day 28 did not show any further increasing effect in those COVID-recovered vaccinees who received a second dose (individuals ‘d’ and ‘e’). Interestingly one COVID-recovered vaccinee who received two doses (individual ‘e’) showed similar anti-S IgG values (3,130 AU/mL) 3–4 weeks after the second dose (at day 51–54) as the COVID-recovered vaccinee who only received a single dose at day 1 and no second dose (individual ‘f’, 3,630 AU/mL).
Fig. 2. (A, B) Time course of anti-Spike immunoglobulin G (IgG) in coronavirus disease (COVID)-recovered vaccinees (individuals d, e, and f). Vaccinations were performed at day 1 and 28. Some participants could not provide samples at each time point (participant ‘e’: day 6–7, day 31–32, and day 37–40; participant ‘f’: day 34–35 and day 37–40). The lacking samples are depicted as Φ.
Once again, we noticed remarkable inter-individual differences within this cohort. For example, at time point 34–35 days, vaccinee ‘d’ showed high levels of anti-S IgG (11,400 AU/mL) whereas vaccinee ‘e’ showed much lower levels (3,850 AU/mL). This amounts to an almost threefold difference between these two individuals which both received two doses.
In both COVID-naive and COVID-recovered vaccinees, anti-S IgG levels decreased after reaching peak levels. Interestingly, COVID-recovered vaccinees reached peak levels at day 10–12 after the first dose and showed decreasing anti-S IgG values at later time points despite a second vaccination at day 28. In contrast, peak levels in COVID-naive vaccinees were reached 1 week after the second dose (at day 34–35 after first vaccination) and decreased afterwards.
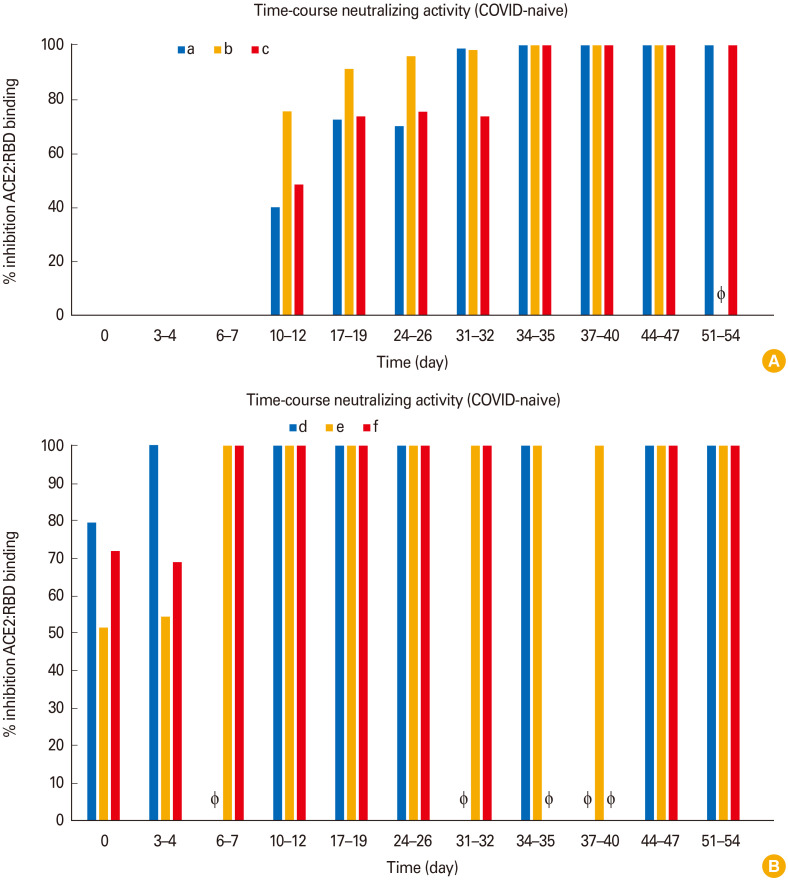
Next, we subjected our samples to a sVNT, which measures the ability of serum samples to disrupt the interaction of the receptor binding domain (RBD) of SARS-CoV-2 and its receptor angiotensin converting enzyme 2 (ACE2) [6]. In brief, 1:10 diluted serum samples were incubated with horseradish peroxidase (HRP)-conjugated RBD as a bait. Subsequently, this mix is added to enzyme-linked immunosorbent assay (ELISA) plates coated with ACE2 and the resulting plate-retained complexes of ACE2 and RBD-HRP are incubated with a colorimetric HRP substrate. The results of this convenient assay—which can be performed without high biosafety requirements—correlates well with results from classical cell culture virus neutralization tests [7]. As shown in Fig. 3A (Fig. 3A depicts COVID-naive vaccinees and Fig. 3B COVID-recovered vaccinees), COVID-naive vaccinees did not develop neutralizing activity until day 10–12 after their first vaccination, subsequently raising to high levels after their second vaccination at day 28.
Fig. 3. Time-course of angiotensin converting enzyme 2 (ACE2):receptor binding domain (RBD) binding inhibition (surrogate virus neutralization test) in coronavirus disease (COVID)-naive (A) and COVID-recovered vaccinees (B). Some participants could not provide samples at each time point (individual ‘b’: day 51–54; individual ‘e’: day 6–7, day 31–32, and day 37–40; individual ‘f’: day 34–35 and day 37–40). The lacking samples are depicted as Φ.
In contrast (and perhaps expected), all COVID-recovered vaccinees (Fig. 3B, lower panel) possessed already measurable neutralizing activity at time point 0 before their first vaccination (as also seen for anti-S IgG, compare Fig. 2A). This is remarkable as their COVID-19 infections had occurred a year ago. Within 4–7 days after their first vaccination, high levels of neutralizing activity (reaching the maximum level of 100% inhibition of the kit with 1:10 diluted samples) were rapidly reached in this group (Fig. 3B). We did not attempt to further titrate our serum samples (beyond the 1:10 dilution proposed by the manufacturer of the kit) to obtain “sVNT titers” because this would be laborious and because the quantitative results of the DiaSorin anti-S IgG assay reportedly correlate well with titers of SARS-CoV-2 neutralizing activity [8].
Taken together, our data revealed a synchronous time-course of rising anti-S IgG and rising neutralizing activity (compare time-course of Figs. 1 and 2 with Fig. 3) in all vaccinees after the first vaccination.
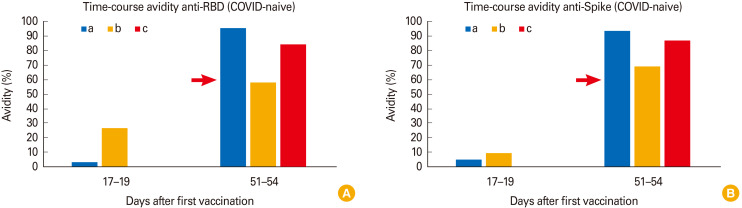
“IgG avidity” describes the strength of binding between IgG and its target epitope. During affinity maturation IgG of high avidity develops and high-avidity IgG is considered as more capable than low-avidity IgG in terms of neutralizing pathogens. Therefore, we next explored the avidity of anti-S IgG and anti-RBD IgG (RBD: the RBD of spike) in our COVID-naive vaccinees (Fig. 4A: anti-RBD IgG; Fig. 4B: anti-S IgG) using the “recomLine SARS-CoV-2 IgG avidity” assay from Mikrogen. In this assay, nitrocellulose test strips coated with recombinant virus proteins (spike and RBD) are incubated with IgG-containing sera from vaccinated individuals. Unbound antibodies are then washed away and the immunoblot is being incubated with secondary antibodies conjugated to HRP, washed, and finally developed by the addition of an HRP-substrate. To address the avidity of the anti-spike or anti-RBD antibodies, the assay is in parallel being performed in the presence or absence of a proprietary solution which disrupts the binding of low-avidity IgG to antigens without affecting the binding of high-avidity IgG to antigens. Subsequently both blots are scanned and densitometric values are compared to each other: a persistent band intensity of >60% despite the presence of the low-avidity disrupting solution (red arrows in Fig. 4) is indicative of high-avidity IgG. We found that all COVID-naive vaccinees started out with low-avidity IgG (day 17–19 after first dose) but later reached high-avidity anti-S IgG after their second dose (at day 51–54 after start of vaccination). Interestingly, no high-avidity anti-Spike IgG anti anti-RBD IgG was found at day 17–19, a time point at which neutralizing activity was present in these sera (compare to Fig. 3A). In addition to this observation, we once again noticed inter-individual differences (for instance compare vaccinee ‘a’ and vaccinee ‘b’).
Fig. 4. (A, B) Avidity-maturation of anti-receptor binding domain (RBD) immunoglobulin G (IgG) and anti-S IgG in coronavirus disease (COVID)-naive vaccinees (individuals a, b, c) between day 17–19 and day 51–54 after vaccination (vaccinations were performed at day 1 and 28). Red arrows indicate the cut-off for high-avidity IgG according to the manufacturer’s instructions (>60%).
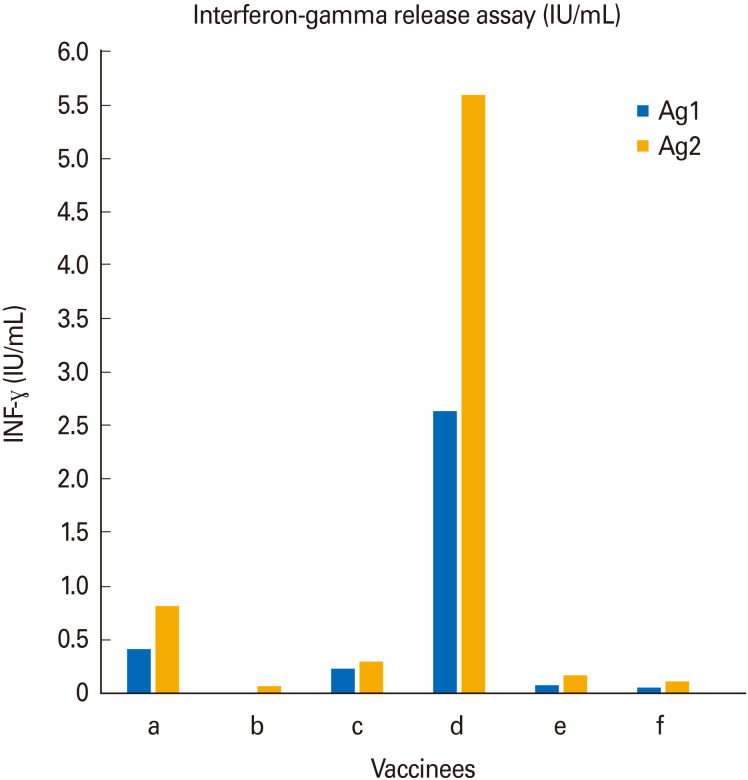
Vaccines induce humoral and cellular immune responses. Next, we examined cellular immune responses to vaccination with mRNA-1273. Interferon-γ Release Assay are commonly used in diagnostic laboratories to measure if pathogen-specific T lymphocytes are present in the blood of individuals [9]. In such assays, blood lymphocytes are stimulated with pathogen-specific peptides and INF-γ release is measured by ELISA. High levels of INF-γ release after stimulation with pathogen-derived peptides indicate established cellular immunity against the pathogen. Therefore, blood from our vaccinees was drawn 12–13 weeks after the start of the vaccination and subjected to an INF-γ Release Assay from Qiagen. This kit uses two specific SARS-CoV-2 peptide pools (Ag1 and Ag2) encompassing the spike protein and designed to stimulate CD4 and CD8 T cells inducing the releases of INF-γ. The kit also contains a positive control antigen which induces robust INF-γ release from T cells. We first confirmed that this internal positive control works: all participants showed robust INF-γ release in response to positive control (data not shown). Subsequently we evaluated the INF-γ response to SARS-CoV-2 antigens Ag1 and Ag2 in our vaccinees. As shown in Fig. 5, we found remarkable inter-individual differences in terms of INF-γ release induced by both antigens ranging from 5,597 IU/mL (Ag2 in vaccinee ‘d’) down to 0 IU/mL (Ag1 in vaccinee ‘b’). Within the cohort of covid-naive vaccinees (individuals a, b, c), vaccinee ‘a’ showed the highest response for both antigens (Ag1: 0.41 IU/mL and Ag2 0.824 IU/mL), vaccinee ‘c’ showed an intermediate response for both antigens (Ag1 0.233 IU/mL and Ag2 0.301 IU/mL) and vaccinee ‘b’ showed a low response for both antigens (Ag1 0 IU/mL and Ag2 0.069 IU/mL). Thus, when comparing the response to Ag2 we found an almost twelvefold difference between vaccinee ‘a’ and vaccinee ‘b’.
Fig. 5. Comparison of interferon-γ (INF-γ) release in vaccinees 12–13 weeks after after mRNA-1273 vaccination. Individuals ‘a’, ‘b’, and ‘c’ refer to our coronavirus disease (COVID)-naive vaccinees, whereas individuals ‘d’, ‘e’, and ‘f’ refer to our COVID-recovered vaccinees. Individual ‘f’ received only one vaccine dose.
Regarding the cohort of covid-recovered vaccinees (individuals d, e, and f), vaccinee ‘d’ showed the highest response (Ag1 2,642 IU/mL and Ag2 5,597 IU/mL) whereas vaccinees ‘e’ and ‘f’ both showed rather low responses (vaccinee ‘e’: Ag1 0.069 IU/mL and Ag2 0.162 IU/mL; vaccinee ‘F’: Ag1 0.06 IU/mL and Ag2 0.111 IU/mL). Thus, when comparing the responses to Ag2 we found an almost thirtyfold difference between vaccinee ‘d’ and vaccinee ‘e’.
Taken together—in a similar way as observed for antibody responses—, we also found large inter-individual differences in terms of cellular responses in our cohort of Moderna vaccinees.
Discussion
During the vaccination campaign in our department, we monitored the time-course of immunological responses to Moderna mRNA vaccination in volunteering COVID-recovered and COVID-naive HCWs. Verifying serological responses to vaccinations seems warranted because the clinical effectiveness of the Moderna vaccine amounts to 94.6% [10] and as non-responders or low-responders to vaccinations are not uncommon in clinical medicine [3]. Accordingly, breakthrough infection despite SARS-CoV-2 vaccination have been reported [11].
Using the DiaSorin anti-S IgG assay and surrogate virus neutralization assay, we found no evidence of “non-responders” (without seroconversion) in our Moderna vaccinees. However, we observed remarkable inter-individual differences between single vaccinated individuals suggesting the existence of high-responders and low-responders to the Moderna vaccine: we found a sixfold difference between highest responder and lowest responder in the COVID-naive vaccine cohort and a threefold difference between highest responder and lowest responder in the COVID-recovered cohort. This difference is probably caused by individual differences regarding responsiveness to the Moderna vaccine as also known for other established vaccines [3]. The problem of low titers after standard vaccination regimes might be overcome by additional booster doses as established for hepatitis B immunization of low-responders [3]. As the magnitude of anti-S IgG levels correlate with protection against emerging variants of concern [12] and because anti-S-IgG levels diminish over time [13], our data seems relevant to health authorities because those vaccinees with rather low “starting levels” of anti-S IgG after their second dose might need a third (booster) dose earlier than those vaccinees with higher anti-S IgG starting levels after their second dose. Thus, determining anti-spike IgG using an assay with a good correlation to neutralizing activity (such as the DiaSorin anti-S IgG assay [8]) appears recommendable to evaluate the level of vaccination-induced acquired humoral immunity after the second dose to obtain a “starting titer set point.” In analogy to the established approach regarding hepatitis B vaccinations [3], a serological evaluation of anti-S IgG levels after completed SARS-CoV-2 vaccination should be discussed at least for high-risk groups such as HCWs or immunosuppressed patients. Encouragingly, research is getting closer to establish cut-offs for convenient serological assays as “correlates of immunity” after successful SARS-CoV-2 vaccination [14].
Besides triggering humoral immune responses, vaccines also induce adaptive cellular responses and cellular immunity against SARS-CoV-2 is emerging as resilient against variants of concern [15,16]. INF-γ Release Assays such as the QuantiFERON test platform from Qiagen are commonly used in clinical laboratories, e.g., to evaluate cellular immunity for Cytomegalovirus or Mycobacterium tuberculosis [17]. We used the QuantiFERON SARS-CoV-2 assay to evaluate cellular responses in our Moderna vaccinees. Similar to the differences observed regarding anti-S IgG levels, we also found remarkable inter-individual differences in terms of INF-γ release. In a similar pattern as observed for anti-S IgG responses, vaccinee ‘d’ showed the highest response levels within the COVID-recovered vaccinee cohort whereas vaccinee ‘a’ showed the highest levels within the COVID-naive cohort. Thus, we also found high-responder and low-responder in terms of cellular responses to vaccination with mRNA-1273.
Another interesting aspect of our study is that COVID-recovered vaccinees and COVID-naive vaccinees can be readily distinguished according to their different serological profiles, because COVID-recovered vaccinees showed rapidly raising anti-S IgG and sVNT values (as surrogate for neutralizing antibodies) within the first week after their first vaccination, whereas COVID-naive vaccinees do not start showing such responses until the second week after first vaccination. Moreover, COVID-naive vaccinees do not show high levels of immune responses until after their second vaccination whereas COVID-recovered vaccinees reach high levels already after a first vaccination. Thus, our data are in line with other recent reports suggesting that COVID-recovered individuals may only need one vaccine dose to mount a sufficient humoral immune response [18,19]. This opinion is supported by our data, because we had the particular opportunity to compare the serological responses of one individual who received two doses of mRNA-1273 (vaccinee ‘f’) and one individual who only received a single dose of mRNA-1273 (vaccinee ‘e’): both vaccinees had similar levels of anti-S IgG at day 51–54 after first dose; moreover, both vaccinees showed similar responses regarding INF-γ release (12–13 weeks after first dose).
Avidity assays for anti-RBD IgG and anti-S IgG demonstrated maturing responses with increasing avidity in COVID-naive vaccinees between 17–19 days and 51–54 days. Thus, at day 17–19 post-vaccination low-avidity anti-RBD IgG/anti-S IgG and neutralizing activity were present in our samples, indicating that low-avidity anti-RBD IgG and low-avidity anti-S IgG exert neutralizing activity. It is very interesting to correlate our serological data of COVID-naive vaccinees with clinical studies exploring the degree of protection after the first dose mRNA-1273 vaccination in COVID-naive individuals. A recent study by Moderna in COVID-naive individuals found a 52% protection against COVID-19 between the first and second dose starting 12 days after the first dose and a maximal protection of 94.6% observed 7 days after the second dose [10]. Intriguingly these clinical findings correlate perfectly with our kinetic serological data in that anti-S IgG and neutralizing activity appear at day 10–12 after the first dose and reach high levels 6–7 days after the second dose (day 34–35) (Figs. 1, 3). As we also found low-avidity anti-S IgG and anti-RBD IgG at day 17–19 after the first dose, our data indicate that neutralizing low-avidity anti-S IgG and neutralizing low-avidity anti-RBD IgG contribute to the partial clinical protection against SARS-CoV-2 infection in the early time window after the first vaccination in COVID-naive vaccinees. However, this intriguing correlation does not rule out other early-onset innate or adaptive immunological mechanisms contributing to the incipient protection in the early time window after a first vaccine dose.
The limitation of our study is a rather small number of participants, whereas the advantages are a high frequency of serum sampling and an in-depth analysis with complementary immunological assays. Further studies will be necessary to support the presented data and conclusions.
Footnotes
No potential conflict of interest relevant to this article was reported.
We thank our volunteers for volunteering to donate blood.
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heininger U, Gambon M, Gruber V, Margelli D. Successful hepatitis B immunization in non- and low responding health care workers. Hum Vaccin. 2010;6:725–728. doi: 10.4161/hv.6.9.12420. [DOI] [PubMed] [Google Scholar]
- 4.Meyer B, Reimerink J, Torriani G, et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg Microbes Infect. 2020;9:2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruttgen A, Cornelissen CG, Dreher M, Hornef MW, Imohl M, Kleines M. Determination of SARS-CoV-2 antibodies with assays from Diasorin, Roche and IDvet. J Virol Methods. 2021;287:113978. doi: 10.1016/j.jviromet.2020.113978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera RA, Ko R, Tsang OT, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59:e02504-20. doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MJ, McIntosh M, Atkinson C, et al. Validation of a commercially available indirect assay for SARS-CoV-2 neutralising antibodies using a pseudotyped virus assay. J Infect. 2021;82:170–177. doi: 10.1016/j.jinf.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonelli F, Sarasini A, Zierold C, et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58:e01224-20. doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrone L, Petruccioli E, Vanini V, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. 2021;27:286. doi: 10.1016/j.cmi.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. MedRxiv [Preprint] 2021 Jan 01; doi: 10.1101/2021.01.26.21250543. [Epub] [DOI] [Google Scholar]
- 13.Beaudoin-Bussieres G, Laumaea A, Anand SP, et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio. 2020;11:e02590-20. doi: 10.1128/mBio.02590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J. Can immune responses predict which vaccines work best? Science. 2021;373:142–143. doi: 10.1126/science.373.6551.142. [DOI] [PubMed] [Google Scholar]
- 15.Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest. 2021;131:e149335. doi: 10.1172/JCI149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarke A, Sidney J, Methot N, et al. Negligible impact of SARS-CoV-2 variants on CD4+ and CD8+ T cell reactivity in COVID-19 exposed donors and vaccinees. Biorxiv [Preprint] 2021 Jan 01; doi: 10.1101/2021.02.27.433180. [Epub] [DOI] [Google Scholar]
- 17.Pieterman ED, Liqui Lung FG, Verbon A, et al. A multicentre verification study of the QuantiFERON(R)-TB Gold Plus assay. Tuberculosis (Edinb) 2018;108:136–142. doi: 10.1016/j.tube.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]