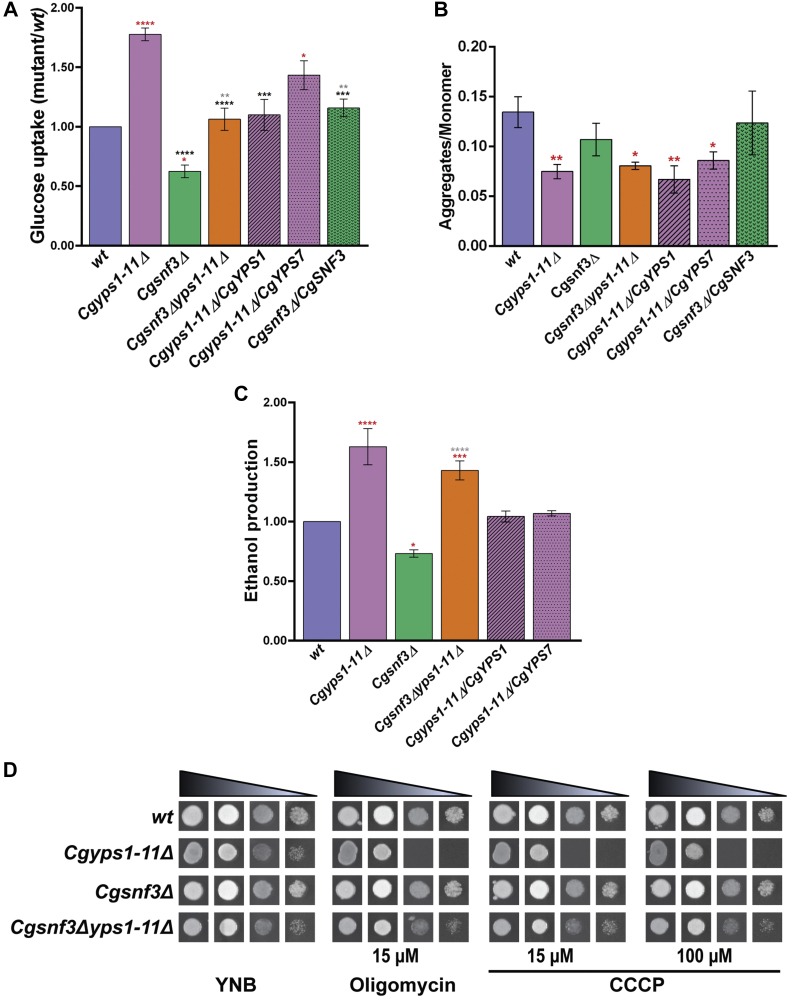
Figure 6.
The Cgyps1-11Δ mutant displays higher glucose uptake.A, uptake of 2-NBDG ([2-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose) in indicated C. glabrata strains, as determined by spectrofluorimetry. Glucose-starved cells were incubated with 2-NBDG (100 μM) for 1 h at 30 °C, and the fluorescence emission was recorded at 540 nm, under excitation at 465 nm. Data (mean ± SEM, n = 3–5) were normalized against the wt fluorescence values (considered as 1.0) and represent fold change in NBDG uptake in mutant strains, compared with the wt strain. Red asterisks denote differences in the glucose uptake between wt and indicated strains, black asterisks denote differences between Cgyps1-11Δ and indicated strains, and gray asterisks denote differences between Cgsnf3Δ and indicated strains. ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001, one-way ANOVA with Tukey’s test. B, JC-1 dye–based assessment of mitochondrial membrane potential. Log-phase cells were stained with JC-1 (20 μM) dye and washed with PBS, and fluorescence of J-aggregates (red) and monomers (green) was recorded at 550 nm excitation/emission 600 nm and excitation/emission 485 nm/535 nm, respectively. The ratio of red fluorescence (J aggregates) to green fluorescence (monomer) was calculated for each strain and plotted. Data represent mean ± SEM (n = 3–4). Red asterisks denote differences between wt and indicated strains. ∗p ≤ 0.05; ∗∗p ≤ 0.01, one-way ANOVA with Tukey’s test. C, ethanol measurement in the culture broth. Ethanol in the culture medium of indicated strains was extracted using dibutyl phthalate, followed by potassium dichromate oxidation of ethanol. The amount of ethanol in the culture medium was calculated from the standard curve, and data (mean ± SEM, n = 3–5) were normalized against ethanol produced by the wt strain (considered as 1.0). Data represent fold change in ethanol production in mutant strains, compared with the wt strain. Red and gray asterisks denote uptake differences between wt and indicated strains, and Cgsnf3Δ and Cgsnf3Δyps1-11Δ mutants, respectively. ∗p ≤ 0.05; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001, one-way ANOVA with Tukey’s test. D, liquid medium–based growth analysis of wt, Cgyps1-11Δ, Cgsnf3Δ, and Cgsnf3Δyps1-11Δ strains in the presence of indicated inhibitors. Cultures were inoculated at an initial A600 of 0.25 and grown in medium lacking (YNB) or containing oligomycin (15 μM) and carbonyl cyanide m-chlorophenylhydrazone (CCCP; 15 and 100 μM). After 12 h, cultures were diluted in PBS, and 3 μl of undiluted and 10-, 100-, and 1000-fold-diluted cultures were spotted on YNB medium. Plates were incubated at 30 °C, and images were captured after 1 day.