Figure 5. Stem cells and cellular origin of metaplasia and cancers.
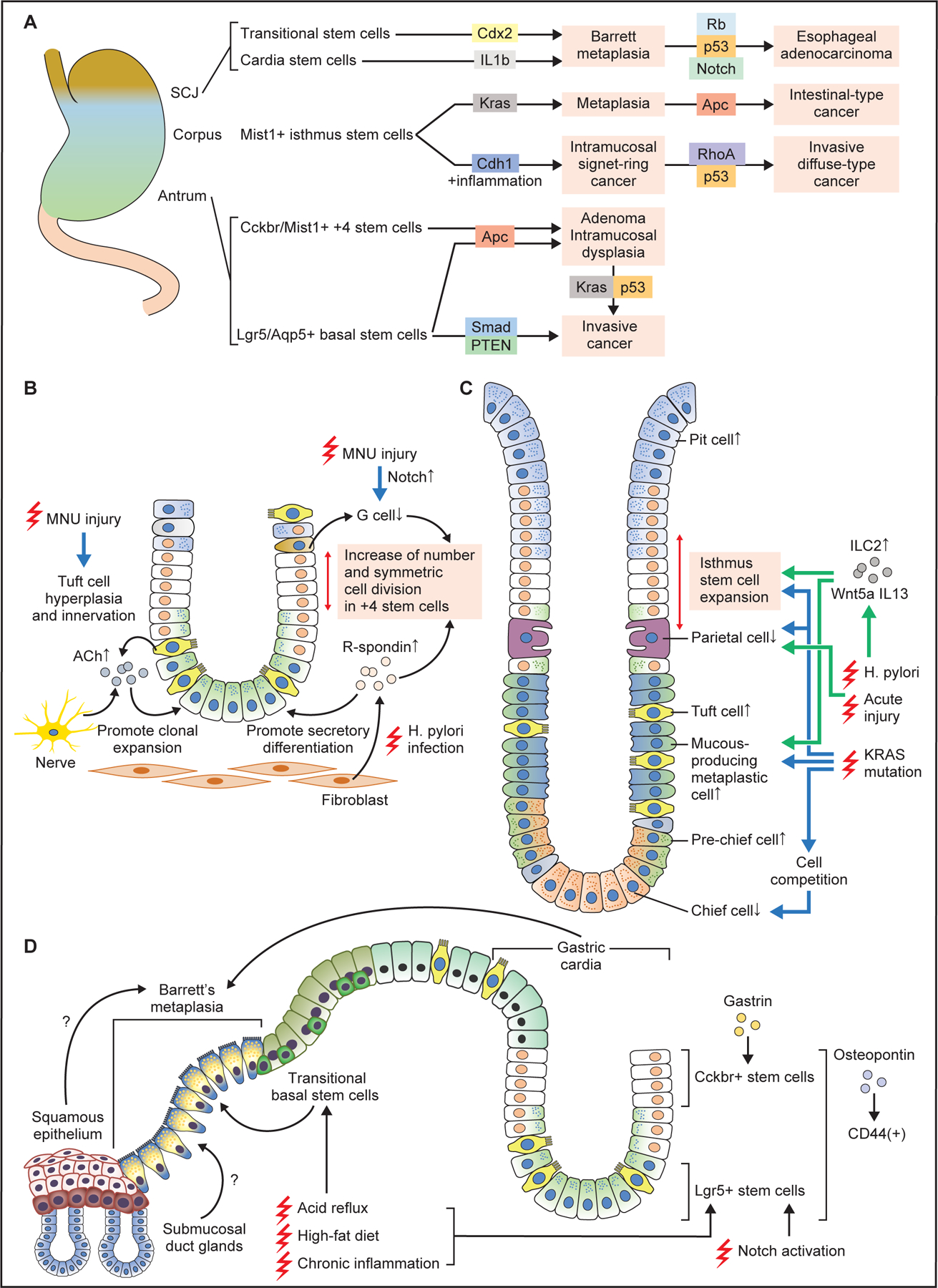
(A) Stem cells in the antrum, corpus, and SCJ give rise to metaplasia and cancer following induction of specific oncogenic mutations. In general, combination of multiple gene mutations causes more advanced, invasive cancers at each site. (B) Changes in antral stem cells and their niche at the early phase of carcinogenesis. Chronic injury by MNU or H. pylori alters stem cell niche, with increase of R-spondin from fibroblasts and acetylcholine (ACh) from nerves and tuft cells, as well as decrease of G cells and gastrin. These contribute to expansion and symmetric cell division of +4 stem cells, and secretory differentiation in Lgr5+ lineage. (C) Cellular changes in the corpus gland during early metaplasia development. Both in the acute and chronic injury models, the numbers of isthmus progenitors, tuft cells, and mucous-producing metaplastic cells are increased, while the numbers of parietal and chief cells are decreased. Wnt5a and IL13 from ILC2s mediate these pathological changes. Induction of Kras mutation in isthmus stem cells causes similar metaplastic changes, while Kras mutation in mature chief cells results in cell competition-dependent loss of chief cells. (D) Schematic image showing Barrett esophagus and carcinogenesis. Stem cells in the gastric cardia glands, including basal Lgr5+ cells and +4 Cckbr+ cells expand and give rise to Barrett metaplasia and cancer in response to chronic injury. High-fat diet and Notch activation promotes the expansion of these cell-derived clones. Transitional basal stem cells also serve as a source of Barrett metaplasia. Esophageal squamous epithelium and submucosal gland ducts might also contribute to Barrett metaplasia development.
