Abstract
Deposition of visceral fat and insulin resistance play central role in the development of non-communicable diseases (NCDs) including obesity, hypertension and type 2 diabetes. However, we shed more light upon the intestines and the kidney as a strong driver of NCDs. Based upon unexpected outcomes of clinical trials using sodium-glucose cotransporter (SGLT) 2 inhibitors to demonstrate their actions for not only body weight reduction and blood glucose fall but also remarkable cardiorenal protection, we speculate that hyperfunction of the intestines and the kidney is one of critical contributing factors for initiation of NCDs. By detecting high amount of glucose and sodium chloride around them by sweet/salt taste sensors, the intestines and the kidney are designed to (re)absorb these nutrients by up-regulating SGLT1 or SGLT2. We designate these hyperfunctioning organs for nutrient uptake as “greedy organs”. The greedy organs can induce NCDs (“greedy organ hypothesis”). SGLTs are regulated by glucose and sodium chloride, and SGLTs or other genes can be “greedy genes.” Regulating factors for greedy organs are renin-angiotensin system, renal sympathetic nervous activity, gut inflammation/microbiota or oxidative stress. Mitigation of organ greediness by SGLT2 inhibitors, ketone bodies, bariatric surgery, and regular lifestyle to keep rhythmicity of biological clock are promising.
Keywords: Sodium-glucose cotransporter, Diabetes mellitus, Chronic kidney disease, Non-communicable diseases, Metabolic syndrome, Obesity
Graphical abstract
Highlights
-
•
We propose the concept of “Greedy Organs” hypothesis as a possible cause of NCDs.
-
•
Clinical implication of greedy kidney is supported by the effect of SGLT2 inhibitors.
-
•
The significance of greedy intestines is suggested by the effect of bariatric surgery.
-
•
The intestines and kidney become hyperactive through upregulation of SGLT1 or 2.
-
•
To mitigate “greedy organs” should be a promising strategy against NCDs.
Abbreviations
- NCD
non-communicable disease
- BP
blood pressure
- DM
diabetes mellitus
- CKD
chronic kidney disease
- CV
cardiovascular
- ATP
adenosine triphosphate
- ROS
reactive oxygen species
- Ang II
angiotensin II
- NP
natriuretic peptide
- ACE
angiotensin converting enzyme
- ARB
angiotensin type 1 receptor blocker
- SGLT
sodium-glucose cotransporter
- IEC
intestinal epithelial cell
- PT
proximal tubule
- GLUT
glucose transporter
- RTC
renal tubular cell
- G6Pase
glucose-6-phosphate
- eGFR
estimated glomerular filtration rate
- RYGB
Roux-en-Y gastric bypass
- NHE
Na+-H+-exchanger
- RAS
renin angiotensin system
- MR
mineralocorticoid receptor
- ENaC
epithelial sodium channel
- EGF
epidermal growth factor
- PAC
plasma aldosterone concentration
- SNA
sympathetic nervous activity
- RSN
renal sympathetic nerve
- HFD
high-fat diet
- SCFA
short chain fatty acid
- FSGS
focal segmental glomerulosclerosis
- β-OHB
β-hydroxybutyrate
1. Introduction
About 70% of cause of death (about 57 million/year) is attributed to “non-communicable disease (NCD)s.” Researches by the World Health Organization and other organizations contributed to increased global awareness of NCDs as important clinical challenges [1].
Obesity induces insulin resistance and causes elevation of blood pressure (BP), postprandial high blood glucose and dyslipidemia which almost simultaneously occur. Postprandial hyperglycemia is often followed by fasting hyperglycemia. These states are called metabolic syndrome, which leads to the onset of type 2 diabetes mellitus (DM), the development of chronic kidney disease (CKD) and the progression of arteriosclerosis. These pathological processes result in renal failure, blindness, stroke, myocardial infarction, heart failure and dementia. In 2003, we defined the series of these events as “Metabolic Domino” (Fig. 1) [2]. Patients with metabolic syndrome are in the early stage of a series of metabolic events before the onset of diabetes. Since the progression of arteriosclerosis starts at this stage, cerebrovascular and cardiovascular (CV) events can occur at any time before or just at the time of the onset of diabetes. CKD is in the midstream of the metabolic domino around at the onset of diabetes. CKD not only causes renal death but also increases the risk of CV events (“cardio-renal connection”) [[3], [4], [5]]. The relationship between cancer and lifestyle-related diseases has been clarified [6]. Cancer has become a major cause of death in diabetic patients. Furthermore, with the advent of the super-aged society, frailty and decreased skeletal muscle mass (i.e., sarcopenia) are another issue of concern [7]. Metabolic Domino, thus, focuses on inter-organ communications and “timing” of event occurrence in NCDs.
Fig. 1.
“Metabolic Domino” (adapted from reference 2)
The collapse of “Metabolic Domino” starts with slight derangement in lifestyle, that is, more food intake and less exercise, which leads to visceral obesity, insulin resistance and causes hyperglycemia, high BP and dyslipidemia. The cluster of these pathological states is called metabolic syndrome. These metabolic abnormalities induce a variety of non-communicable diseases and organ dysfunctions, that are the cases of poor quality of life and short healthy life expectancy.
ASO =
arteriosclerosis obliterans. ED =
erectile dysfunction.
In NCDs or Metabolic Domino, it is recognized that the common cause of series of events is obesity or visceral fat deposition. In this review article, however, we shed more light on altered function of the intestines and the kidney, which are a strong driver of the sequence of such clinical events.
2. The intestines and the kidney: critical organs for NCDs
As a common pathophysiology in NCDs, we have focused on abnormalities in mitochondria. Mitochondria produce adenosine triphosphate (ATP) needed for living activity. A decrease in mitochondrial function not only leads to decreased ATP production but also increases reactive oxygen species (ROS). Decreased ATP production and increased ROS induce organ dysfunction and aging [8].
We previously reported that angiotensin II (Ang II) reduced the content of mitochondria in the skeletal muscle and deteriorated glucose tolerance [9]. In contrast, natriuretic peptide (NP)s, antagonizing hormones to Ang II, and cyclic guanosine monophosphate, the second messenger of NP, stimulated mitochondrial biogenesis, and prevented obesity [10,11]. Both angiotensin converting enzyme (ACE) inhibitors and angiotensin type 1 receptor blocker (ARB)s are considered to improve glycemic control [12,13], and suppress new onset of diabetes [13,14]. Angiotensin receptor-neprilysin inhibitor is reported to lower hemoglobin A1c level in diabetics [15].
Organs rich in mitochondria are the intestines and the kidney. Both organs require high ATP consumption for exerting their biological action of “(re)absorption” of needed materials into the body from the environment. Although renal filtering through glomeruli does not require energy, reabsorption from renal tubules requires a considerable amount of energy.
We believe that it is safely stated that the intestines and the kidney are involved in the regulation of the function of mitochondria in all organs. The intestines are responsible for the absorption of glucose and fat (and amino acids), which serve as the source material for ATP production, and the kidney plays a role in delivering these nutrients and oxygen (the fuel for ATP production) effectively to the whole body by regulating BP through promoting sodium (NaCl) retention and renin secretion.
3. Hypothesis of “Greedy Organs”: the intestines and the kidney are greedy for sugar and salt
It has been believed that NCDs are induced by dysfunction/failure of organs. However, we have introduced the hypothesis of “greedy organs” as a cause of NCDs.
The word “greedy” has the following three meanings: 1) wanting to eat or drink more than one can reasonably consume (in relation to eating behavior) 2) immoderately desirous of acquiring e.g. wealth (with bad meaning) 3) ardently or excessively desirous (with good meaning). We think that during the course of “Metabolic Domino”, the intestines and the kidney hyper-function as greedy organs, with all of these three meanings. We use the word, “greedy”, in an association with a slang phrase, “greedy-guts”, which means big eater.
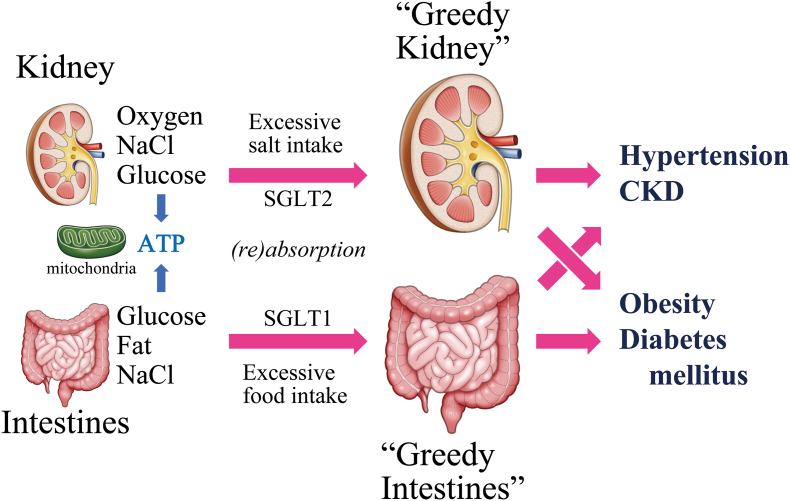
In brief, reabsorption of sodium (NaCl) by the “greedy” kidney leads to hypertension/CKD, and the “greedy” absorption of glucose and fat by the intestines causes obesity/diabetes. Importantly, at the same time, excessive glucose release and/or production by the “greedy” kidney also causes diabetes and “greedy” absorption of sodium by the intestines also causes hypertension, forming a “crossing” relationship between the two organs and two disease entities (Fig. 2) [16].
Fig. 2.
“Greedy organs” and lifestyle-related diseases
The kidney and intestines absorb excessive nutrients for the production of ATP in mitochondria and become “greedy.” Excessive salt intake through SGLT2 makes the kidney greedy to induce hypertension/CKD. Excessive food intake through SGLT1 make the intestines greedy to induce obesity/DM. There is a “crossing” relationship between “greedy organs” and lifestyle-related disease, that is, “greedy kidney” can cause obesity/DM and “greedy intestines” can cause hypertension/CKD.
ATP =
adenosine triphosphate. CKD =
chronic kidney disease. SGLT =
sodium-glucose cotransporter.
Because greedy organ hypothesis is a concept for dissecting pathophysiology of NCDs from the viewpoint of “(re)absorption of nutrients,” we do not include the brain or the liver, which utilizes or processes them, into greedy organs, although they significantly contribute to salt/glucose metabolism.
We came up with the above hypothesis based on the findings of our study concerning the effect of sodium-glucose cotransporter (SGLT)2 inhibitors [17,18]. SGLT is a cotransporter that absorbs glucose and sodium simultaneously. SGLT1 is predominantly expressed in intestinal epithelial cell (IEC)s, whereas SGLT2 is predominantly expressed in renal tubular epithelial cells. Both make simultaneous absorption of glucose and sodium into the body.
Even under pathophysiological circumstances with excessively high glucose/sodium concentration, the intestines and the kidney are to (re)absorb these nutrients more than metabolized or stored under the existing conditions.
Glucose filtered by the kidney is reabsorbed by SGLT2, highly expressed in the proximal tubule (PT), and released into the bloodstream by glucose transporter (GLUT)2 (Fig. 3) [19,20]. Our experiment [18] using cultured renal tubular cell (RTC)s showed that the higher the concentration of glucose in the extracellular media, the greater the reabsorption of glucose by the RTCs due to increased SGLT2 expression. An in vivo experiment using db/db diabetic mice revealed that RTCs in hyperglycemia reabsorb a high amount of glucose leaked into the urine due to, in part, increased SGLT2 expression in RCTs, in spite of high glucose levels [18]. In other words, RTCs are “greedy”.
Fig. 3.
Possible coordination among GLUT2, SGLT2, and Na+-K+-ATPase for effective glucose reabsorption from the urine
When GLUT2 in the proximal tubule senses glucose in basolateral side, importin-α1 and HNF-1α dissociate from the GLUT2 and translocate into the nucleus, then SGLT2 expression increases. SGLT2 at the enhanced level transports more sodium and glucose simultaneously into the renal tubular cells using energy generated by Na+-K+-ATPase. Then, glucose and sodium are transported into the bloodstream through GLUT2 and Na+-K+-ATP channel, respectively.
ADP =
adenosine diphosphate. ATP =
adenosine triphosphate. Glu =
glucose. GLUT =
glucose transporter. HNF =
hepatocyte nuclear factor. SGLT =
sodium-glucose cotransporter.
According to a series of studies using IECs, the intestines are demonstrated to take up nutrients greedily [21,22]. It has also been reported that the intestines, like the kidney, absorb high amount of glucose by increasing SGLT1 expression when they detect sweet substances through sweet-responsive type 1 taste receptor 3 (T1R3) and the G protein gustducin [23].
In addition, when glucose concentration is high around the environment, the kidney additionally makes more glucose (renal gluconeogenesis). In humans, gluconeogenesis occurs in the liver and the kidney, both of which possess the key enzyme of gluconeogenesis, glucose-6-phosphate (G6Pase) [24]. Hepatic gluconeogenesis maintains blood glucose levels during starvation periods such as sleep time to prevent hypoglycemia. However, the importance of renal gluconeogenesis has not been clarified well. In fact, renal gluconeogenesis is regulated in rather opposite direction to that of hepatic gluconeogenesis.
Diabetes is associated with not only increased glucose reabsorption due to the up-regulation of SGLT2, but also increased renal gluconeogenesis [25]. Thus, the relationship between renal gluconeogenesis and glucose reabsorption seems to be synergistic, not complementary.
4. Clinical implications of “Greedy Organs”: sugar and salt are bad companions for NCDs
We believe that attenuation of hypermetabolism in the kidney after the administration of SGLT2 inhibitors maintains renal cell function and prevents the exhaustion of the kidney. “The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME)” published in 2015 [26] supports this hypothesis. Effects being independent of lowering blood glucose level may contribute to the unexpected clinical outcomes, based on the following findings: a) overall mortality decreased a few months after the administration of SGLT2 inhibitors; b) a sub-analysis showed a significant contribution of the decrease of heart failure to the reduction of the overall mortality; and c) no reduction in the mortality of stroke was observed. Therefore, SGLT2 inhibitors can function not only as an anti-metabolic agent but also as a CV agent. The sub-analysis of the EMPA-REG OUTCOME focusing on renal events published in 2016 (EMPA-REG Renal Outcome) [27] showed that SGLT2 inhibitor, compared with placebo, reduced the risk of development or worsening of renal events by 39%. This effect, however, cannot be explained by diuretic effects.
EMPA-REG OUTCOME trial included type 2 diabetic patients with both preserved and modestly reduced kidney function. Administration of SGLT2 inhibitor led to an initial acute decline in estimated glomerular filtration rate (eGFR) of about 3 mL/min/1.73 m2. This initial dip in eGFR is considered to be caused by reduction of enhanced intraglomerular pressure, through tubule-glomerular feedback in the “greedy kidney.” Improved glomerular hyperfiltration, was followed by a marked slower decline in eGFR compared with those allocated to placebo during longer-term treatment, then finally leading to the risk reduction of renal dysfunction by 39% compared with placebo [27].
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial showed that reno-protective effect of SGLT2 inhibitor can be extended to non-diabetic CKD [28]. Moreover, in a sub analysis of DAPA-CKD using the same primary endpoint, dapagliflozin showed a significant beneficial effect in 270 IgA nephropathy patients [29].
Reno-protective effect of SGLT2 inhibitor in non-diabetic CKD could be interpreted that residual nephrons in reduced kidney mass by various renal diseases suffer from the overload of filtered glucose and sodium, which they would do overwork to greedily reabsorb these nutrients as much as possible, in the place of destroyed nephrons. The administration of SGLT2 inhibitors can alleviate the greediness and exhaustion of the residual nephrons leading to reno-protection regardless of the cause of CKD.
Clinical trials concerning the “greedy” intestines are scarce. Long term follow-up study (median duration 6.3 years) showed that obese patients (n = 1,724, mean body mass index 46.5 kg/m2) who underwent Roux-en-Y gastric bypass (RYGB) surgery showed significantly lower incidence of major CV events (composite of stroke, myocardial infarction, and congestive heart failure) and congestive heart failure as compared with matched unoperated control (n = 1,724) [30].
We recently demonstrated that salt also increases the expression of SGLT1 in the intestines [31]. High salt intake for 8 weeks to rats increased the expression of SGLT1 along with Na+-H+-exchanger (NHE)3 through the up-regulation of intestinal renin angiotensin system (RAS). There is another report to demonstrate that salt could activate SGLT1. RYGB in minipigs reduced the glucose uptake in the bile-deprived alimentary limb (AL) in the intestine. When bile, which contains high concentration of sodium, or salt (NaCl) itself was added to AL, glucose uptake was restored which was blocked by the SGLT1 inhibitor phlorizin, suggesting SGLT1 activation by salt [32].
5. “Greedy genes”
As mentioned above, SGLT1 or SGLT2 behaves to absorb sugar (glucose) and salt (sodium chloride) as much as possible (“greedily”) by increasing its gene expression with elevated ambient concentration of glucose or sodium, leading to excessive absorption of these nutrients. We would like to call such genes as “greedy genes”, which contribute to the formation of greedy organs (Table 1).
Table 1.
Possible pathological states/diseases associated with “greedy organs” and “greedy genes”.
| Pathophysiological states/diseases | Related “greedy genes” |
|---|---|
| Obesity | SGLT1 [32] |
| Glucose intolerance/Diabetes Mellitus | SGLT2 [18,26,33,34], SGLT1 [32] |
| Hypertension | |
| Essential hypertension | MR [37], NHE [43], |
| Salt-sensitive hypertension | SGLT1 [31], MR [35,36], ENaC [42], NHE [45] |
| “MR-associated hypertension” | MR [41] |
| CKD (especially at early phase) | |
| Diabetic | SGLT2 [27,28] |
| Non-Diabetic | SGLT2 [28,29] |
| Congestive heart failure | SGLT2 [26], SGLT1 [30] |
| Cancers | |
| Pancreas | SGLT2 [74] |
| Kidney | SGLT2 [75] |
| Colon | SGLT1 [76,77] |
CKD = chronic kidney disease. ENaC = epithelial sodium channel. MR = mineralocorticoid receptor. NHE = sodium-hydrogen exchanger. SGLT = sodium-glucose cotransporter.
Sugar increases SGLT2 expression in the kidney. It was shown that human exfoliated PT epithelial cell (HEPTEC)s isolated from type 2 diabetic patients expressed more SGLT2 and exhibited increased glucose uptake compared with HEPTECs from healthy individuals [33]. Increased expression of SGLT2 mRNA and protein was shown in the kidneys of type 2 diabetics with nephropathy [34] and db/db diabetic mice [18].
As the list of “greedy genes”, we want to add the genes encoding mineralocorticoid receptor (MR) and epithelial sodium channel (ENaC), the target of MR, along with NHE.
It is reported that salt increases expression/activity of MR. High-salt diet activates Rac1, a member of the Rho family GTPase, in the kidneys in rodent models of salt-sensitive hypertension, leading to high BP and renal injury through an MR-dependent pathway [35], and that Rac1 directly activated MR signal transduction both in vitro and in vivo [36]. We demonstrated that intestinal MR is involved in sodium absorption and BP regulation [37], and that increased activity of intestinal MR can cause hypertension and CKD.
Sugar also increases the expression/activity of MR. We demonstrated that high glucose stimulated MR transcriptional activity and increased MR protein levels through decreased ubiquitination of MR protein in HEK293-MR cells [38], which are modified HEK293 cells expressing human MR stably [39]. We also reported that epidermal growth factor (EGF) increased MR transcriptional activity through EGF receptor and increased protein level by counteracting MR ubiquitination in vitro [40].
We proposed the concept of “MR-associated hypertension [41],” which was hypertension induced by MR overactivation irrespective of plasma aldosterone concentration (PAC). Even with low PAC, MR can be activated by increased MR gene transcription, increased MR sensitivity, or MR stabilization. MR-associated hypertension is often accompanied by NCDs, such as obesity and DM, with elevated insulin resistance and high plasma glucose level.
ENaC plays important roles for the regulation of sodium reabsorption in the kidney and the maintenance of normal BP. It was reported that the expression of α, β, γ-subunits of ENaC, and serum- and glucocorticoid-inducible kinase 1 mRNA was elevated by high sodium diet in Dahl salt-sensitive rats [42]. Hence, the expression of the genes encoding ENaC is upregulated by sodium, which results in the enhanced absorption of more salt and development of salt-sensitive hypertension.
As for NHE, increased expression of NHE is suggested to cause sodium retention and hypertension. In platelets from the patients with essential hypertension [43] and lymphocytes from spontaneously hypertensive rats [44], the activity of NHE was increased. Transgenic mice overexpressing NHE in renal tubules showed significantly decreased urinary excretion of water and Na+, and significantly elevated systolic BP after salt loading [45].
Overactivation of “greedy genes” accelerates an absorption of bad companions, that is, sugar and salt, leading to insulin resistance, hypertension/DM, CKD and chronic heart failure (Fig. 2).
6. How to taste sugar and salt in Greedy Organs
Sugar increases SGLT1 expression in the intestine. The sweet taste receptor subunit T1R3 and the taste G protein gustducin, expressed in enteroendocrine cells, act as sugar sensors. Dietary sugar increased SGLT1 mRNA and protein expression, and glucose absorption is increased in wild-type mice, but not in knockout mice lacking T1R3 or α-gustducin [23].
Diabetes is generally associated with increased glomerular filtration rate and increased tubular sodium reabsorption [46,47]. The energy that drives these processes is generated largely by the enzyme Na+-K+-ATPase in RTCs, which creates an inward negative membrane potential and Na+-gradient [20]. Using this Na+-gradient, SGLT2 reabsorbs glucose with sodium. On the basolateral side of PT cells, GLUT2 transports glucose reabsorbed from the urinary lumen by SGLT2 to the interstitial space and peritubular capillaries in the kidneys. Thus, the coordination among GLUT2, SGLT2, and Na+-K+-ATPase is crucial for effective glucose reabsorption from the urine. As an underlying mechanism of this coordination, we revealed that GLUT2 works as a glucose sensor and regulator of SGLT2 expression by an in vitro study using LLC-PK1 porcine renal epithelial cells [18]. When GLUT2 in the PT was stimulated by high glucose in basolateral side, importin-α1 and hepatocyte nuclear factor-1α, which were bound to GLUT2, dissociated and translocated into the nucleus, then increased SGLT2 expression (Fig. 3).
In mice and rats, ENaC, one of “the greedy genes”, has been proposed as a candidate of salt taste receptor [48,49]. ENaC might be also involved in human sodium detection and salt preference although the relevant mechanisms are not clear [50].
7. Regulating factors for Greedy Organs
7.1. RAS
Using the kidney biopsy specimen of non-diabetic patients with proteinuric glomerular diseases, SGLT2 mRNA level was shown to be significantly associated with angiotensinogen, renin, and ACE mRNA levels [51], which suggests the involvement of intra-renal RAS in SGLT2 expression. Ang II increased SGLT2 expression level in an in vitro study using the human immortalized renal PT cell line (HK-2). Transgenic mice overexpressing angiotensinogen in their renal PT cells and wild type mice with a single injection of Ang II showed increased SGLT2 expression levels in the PT. In an in vitro study using porcine endothelial cells, Ang II increased SGLT1 and SGLT2 protein expression, produced oxidative stress, resulting in increased senescence-associated beta-galactosidase activity and endothelial dysfunction, which were inhibited by SGLT1/SGLT2 inhibitors and ARB [52].
7.2. Renal sympathetic nervous activity (SNA)
De Oliveira et al. [53] reported that renal SNA was significantly increased and splanchnic SNA was significantly decreased in DM rats induced by streptozotocin. Bilateral renal denervation of these rats reduced hyperglycemia, glycosuria, albuminuria, and SGLT2 mRNA expression in the kidney, normalized splanchnic SNA, and improved cardiac baroreflex sensitivity which was impaired in these rats. The result indicates that SGLT2 expression is directly/indirectly (through high glucose level) up-regulated by renal SNA.
Conversely, SGLT2 activity may modulate SNA. Alloxan-induced diabetic rabbit showed increased blood glucose, elevated BP, and exaggerated renal sympathetic nerve (RSN) response to lowering BP, although heart rate and RSN activity itself were not changed [54]. Empagliflozin reduced this exaggerated RSN response to the level in non-diabetic rabbits, effectively normalizing the baroreflex. The inhibitory effect of empagliflozin on RSN activity was also observed in rat kidney injury model without diabetes [55]. Taken together, empagliflozin mitigated the exaggerated RSN activity, in other words, mitigated the greedy kidney with or without diabetes.
7.3. Gut inflammation and alteration of gut microbiota
Using macrophage-specific chemokine receptor 2-knockout and IEC-specific tamoxifen-inducible Ccl2-knockout mice, we previously reported that a high-fat diet (HFD) increased Ccl2 expression in IECs that leads to the recruitment of pro-inflammatory macrophages, increased gut permeability with inflammation and insulin resistance in the adipose tissue [56]. It is reported that gastrointestinal inflammation of mice, which is induced by dextran sodium sulfate and pro-inflammatory cytokines, increased SGLT1 in IECs that leads to glucose handling dysregulation [57]. Taken together, it is speculated that gut inflammation induced by HFD might increase the expression of SGLT1 and make the gut greedy for sugar.
The greedy intestines might be modulated by gut microbiota. The serum concentration of metformin in type 2 diabetic patients was demonstrated to be correlated with that of short chain fatty acid (SCFA) (butyric acid and propionate acid) [58]. Metformin shifted gut microbiota composition and increased several SCFA-producing microbiotas [59]. When SCFA was supplemented to total parenteral nutrition, GLUT2 mRNA and protein was upregulated in the jejunum, resulting in increased jejunal uptake of L-glucose and lauric acid [60]. Conversely, Canagliflozin, which is a not only SGLT2 but also modest SGLT1 inhibitor, increased cecal SCFA and reduces plasma uremic toxins in mice [61].
7.4. Oxidative stress
It was demonstrated that insulin increased tubular SGLT-2 expression and reactive oxygen species in a dose-dependent manner, and stimulated glucose entry into cultured PT cells obtained from the human kidney [62]. Because an anti-oxidant N-acetylcysteine completely blocked these effects of insulin, this observation could be interpreted that SGLT2 expression was regulated via oxidative stress generation.
Empagliflozin was shown to improve diabetic myocardial structure and function, decreased myocardial oxidative stress, and ameliorated myocardial fibrosis [63]. In experiments using type 2 diabetic mice, ipragliflozin reduced urinary albumin excretion, inhibited glomerular hypertrophy and relieved mitochondrial injury by reducing NADPH oxidase 4 expression [64]. In this study, ipragliflozin exerted reno-protective effects by reducing oxidative stress in tubular epithelial cells and glomerular podocytes.
Taken together, increase of oxidative stress would induce SGLT2 expression, followed by increase of uptake of glucose and sodium, and increase of sodium and glucose uptake induces oxidative stress, which forms a vicious cycle.
In obese high-fat diet-fed rats, the expression levels of renal gluconeogenic enzymes including G6Pase were elevated, and oxidative stress markers in the kidney were increased, both of which were decreased by SGLT2 inhibitor [65]. As both apocynin, a selective NADPH oxidase inhibitor, and tempol, a superoxide radical scavenger, inhibited renal gluconeogenesis in vitro and in vivo [66], it is possible that augmented oxidative stress induced by up-regulation of SGLT2 might stimulate renal gluconeogenesis.
8. Time-course of Greedy Organs (hyperfunction to hypofunction/failure)
Although we reported the up-regulation of SGLT2 in PTs of db/db mice [18], the existing literature is inconsistent regarding SGLT2 expression in the diabetic kidney. On one hand, mRNA and protein expressions of SGLT2 increased in kidney biopsy specimen of type 2 diabetics with nephropathy [34]. On the other hand, it was reported that SGLT2 mRNA expression in PTs was lower in type 2 diabetic patients than nondiabetic control [67,68]. A study using human kidney biopsy tissue of glomerulonephritis (minimal change disease, membranous nephropathy, focal segmental glomerulosclerosis (FSGS), IgA nephropathy) showed that tubular SGLT2 mRNA expression was correlated positively with eGFR and negatively with interstitial fibrosis [68]. It means that when eGFR is high, SGLT2 is highly expressed and the kidney can become greedy. While the renal function is decreasing with the decrease of nephron number, SGLT2 activity might be enhanced with greediness in residual nephrons, however, after the significant progression of tubular fibrosis, SGLT2 expression comes to decrease and kidney cannot get greedy anymore.
It was reported that 10 nondiabetic FSGS patients did not show significant change in eGFR nor 24-h urine protein excretion after 8-week administration of dapagliflozin [69]. Because renal parenchymal SGLT2 mRNA expression decreased in FSGS patients compared with controls, this ineffectiveness of dapagliflozin might be due to the downregulation of SGLT2. The sensitivity analysis of this study showed that dapagliflozin decreased urinary protein evidently among patients with low urinary protein excretion at the baseline. Therefore, SGLT2 inhibitor might exerts reno-protective effects only when renal tubular function is maintained. The greediness of the kidneys might be achieved by active tubules.
It could be postulated that excessive greediness and too much accumulation of nutrients might lead to hyperfunction initially, and then hypofunction of the organs, finally resulting in organ failure.
9. Cancers and Greedy Organs
Comprehensive investigation of the relationship between diabetes and cancer risk using eight Japanese cohort studies (>330,000 subjects) revealed that DM was associated with 20% increased risk of total cancer incidence [6]. In addition, a significantly increased risk was observed for cancers at specific sites, such as the colon, liver, pancreas, and bile duct, all of which associated with nutrient digestion and absorption. Positive link between renal cell carcinoma and diabetes was also reported [[70], [71], [72]]. Greedy organs of diabetic patients might be vulnerable to cancer (Table 1). Since cancer cells require high amounts of glucose for uncontrolled proliferation, cancer cells exhibit altered metabolism, characterized by a transition from oxidative phosphorylation to glycolysis (Warburg effect) [73]. It is plausible to speculate that the threshold of cancer cells to become greedy for sugar might be lower than that of normal cells.
The functional expression of SGLT2 was demonstrated in human pancreatic and prostate adenocarcinomas [74]. SGLT2 inhibitor reduced tumor growth and improved survival rate in a xenograft mouse model of pancreatic cancer [74]. Kuang et al. showed higher expression levels of SGLT2 in human renal cell carcinoma cell lines (ACHN, A498, and Caki-1 cells) as compared with HK-2 cells [75].
Guo. et al. reported that the overexpression of SGLT1 and EGF receptor was related to higher clinical stages and poor prognosis of the patients with colorectal cancer [76]. Weihua et al. [77] also reported that SGLT1 protein and EGF receptor were overexpressed in various human cancer cell lines including colon cell line.
10. How to mitigate Greedy Organs
10.1. SGLT2 inhibitors
Findings from EMP-REG Renal Outcome [27] showed that the administration of SGLT2 inhibitors led to better clinical outcomes. Therefore, SGLT2 inhibitors are expected to provide a new mode of reno-protection. This may be true for heart failure as well. SGLT2 inhibitors currently draw much attention as possible novel cardiorenal protective agents. It is speculated from an experiment using rat nephrons that SGLT2 blockade in diabetes lowers cortical oxygen consumption and raises medullary oxygen consumption, leading to the respite of vulnerable PT cells [78].
SGLT2 inhibitors are, thus, expected to be the promising agents to suppress greedy organs and exert critical organ protection. However, there would be some caution. When SGLT2 inhibitors became commercially available between 2013 and 2014, many patients treated with SGLT2 inhibitors were hospitalized due to severe ketoacidosis. Therefore, the U.S. Food and Drug Administration issued a warning to avoid the use of SGLT2 inhibitors in patients with type 1 diabetes who are at higher risk of ketoacidosis [79]. A possible cause of ketoacidosis is postulated that potential hypoglycemia after the administration of SGLT2 inhibitors is sensed by the kidney, leading to the activation of renal vagal afferent nerves and sympathetic nerves, which stimulates lipolysis in the adipose tissues and produces ketone bodies (Graphic abstract).
10.2. Ketone bodies
Regarding the beneficial effects related to SGLT2 inhibitors, ketone bodies produced by fatty acid oxidation are drawn attention. Among various hypothesis explaining the reno-protective effects of SGLT2 inhibitors, the “thrifty substrate” hypothesis [80] may support our “greedy” kidney hypothesis. Energy production in humans requires glucose and fatty acids as the main substrates. Although fatty acids have high energy-producing capacity, high amount of oxygen is needed to burn them. An analysis of ATP production with the same amount of oxygen showed higher ATP production level by glucose compared with that by fatty acids [81]. The heart constantly requires high level of energy and normally produces energy almost exclusively by fatty acid oxidation [82]. However, the heart in ischemia uses glucose as the alternate energy substrate due to reduced oxygen supply. Ketone bodies have relatively high energy value with moderately low oxygen consumption, compared to fatty acids and glucose. The administration of SGLT2 inhibitors leads to increased blood ketone bodies. When the heart and kidney use ketone bodies more as a substrate for energy production, SGLT2 inhibitors may increase the efficiency of energy acquisition, reducing cardiac and renal events, resulting in organ protective effects [83]. These hypotheses are consistent with our idea that SGLT2 inhibitors mitigate the greedy organs. A report from Mizuno et al. [84] demonstrated that human diabetic heart uses less glucose and more ketone bodies as an energy source than non-diabetics.
β-hydroxybutyrate (β-OHB), which is an endogenous ketone body, is expected to be an anti-ischemic molecule, and its strong protective effects were shown in the heart, brain, and liver of rodents. We found that the administration of β-OHB to mice attenuated renal ischemia-reperfusion injury by blocking pyroptosis [85]. Other probable mechanisms of anti-ischemic effects are; reduction of oxidative stress, mitochondrial protection, or enhanced autophagy [86]. An untargeted metabolomic approach demonstrated lower circulating levels of β-OHB in high salt-fed hypertensive rats. Moreover, the rescue of low β-OHB levels by nutritional supplementation of its precursor attenuated salt-sensitive hypertension in rats [87].
10.3. Bariatric surgery
To explore possible factors to contribute to the glucose- and BP-lowering effects of RYGB is one of active research fields with many and diverse candidate mechanisms. Among them, alteration of gut microbiota is considered to play some roles. It was demonstrated in 2013 that in mice, microbiota was altered by RYBG and transplantation of altered microbiota to germ free mice induced body weight reduction [88]. We reported increased levels of incretins and bile acid after bariatric surgery with improved glucose tolerance along with change of gut microbiota in humans [89]. Since in 7.2., we describe that alteration of gut microbiota is one of the regulating factors for greedy organs, bariatric surgery can serve, to a certain extent, to mitigate the greedy intestine.
10.4. Regular lifestyle rhythm
Possible relationship between clock genes and greedy genes have been suggested. There was a circadian rhythmicity in intestinal glucose absorption, which was mediated via diurnal alterations in SGLT1 expression in rat jejunum [90,91]. Circadian clock proteins Per1and Clock were detected at promoters of NHE3 and SGLT1. Per1 was shown to regulate the expression of NHE3 and SGLT1 in PT cells of mice at the level of transcription [92]. Thus, the clock genes might regulate glucose absorption from the intestine via greedy genes.
HFD attenuated the circadian rhythm of feeding and locomotor activity, and reduced the amplitude of mRNA expression levels of Clock, Bmal1, and Per2 in the hypothalamus, liver, and fat in mice [93]. Whereas, time-restricted feeding of HFD, without caloric restriction, magnified the oscillation of the mRNA expression of circadian clock genes such as Per2 and Bmal1, improved glucose metabolism, and prevented obesity in mice [94].
It is not clear whether the disturbed rhythmicity of glucose absorption from the intestines increase the expression or activity of the “greedy genes.” Nevertheless, regular lifestyle is necessary to keep biological clock in shape, leading to a reduction of insulin resistance and prevention of NCDs.
11. Conclusion
We believe that greedy organs including the intestines and the kidney can be one of critical contributing factors for NCDs, such as type 2 diabetes and hypertension, and that a new mode of therapy that mitigates the greedy organs is expected to be promising for NCDs. However, further investigation is required to develop a sophisticated method that effectively mitigates the greedy organs.
Funding
HI was supported by Grants-in-Aid for Scientific Research (A), Challenging Research (Exploratory), and Advanced Research and Development Programs for Medical Innovation (AMED-CREST).
Author contributions
HI conceptualized this review article. Both authors performed the literature review; analyzed and interpreted the data; wrote and revised the manuscript.
Declaration of competing interest
HI: Lecture fees: MSD, Mitsubishi Tanabe pharma corporation, Nippon Boehringer Ingelheim Co., Ltd, ONO Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd.
References
- 1.NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet. 2018;392(10152):1072–1088. doi: 10.1016/S0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 2.Itoh H. [Renin-angiotensin system and metabolic domino] Nihon Rinsho. 2005;63(Suppl 3):180–187. [PubMed] [Google Scholar]
- 3.Ito S. Cardiorenal connection in chronic kidney disease. Clin Exp Nephrol. 2012;16(1):8–16. doi: 10.1007/s10157-011-0493-2. [DOI] [PubMed] [Google Scholar]
- 4.Keith D.S., Nichols G.A., Gullion C.M., Brown J.B., Smith D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 5.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Sasazuki S., Charvat H., Hara A., Wakai K., Nagata C., Nakamura K., Tsuji I., Sugawara Y., Tamakoshi A., Matsuo K., Oze I., Mizoue T., Tanaka K., Inoue M., Tsugane S. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104(11):1499–1507. doi: 10.1111/cas.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 8.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuishi M., Miyashita K., Muraki A., Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes. 2009;58(3):710–717. doi: 10.2337/db08-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsuishi M., Miyashita K., Itoh H. cGMP rescues mitochondrial dysfunction induced by glucose and insulin in myocytes. Biochem Biophys Res Commun. 2008;367(4):840–845. doi: 10.1016/j.bbrc.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., Sone M., Yamahara K., Taura D., Inuzuka M., Sonoyama T., Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iimura O., Shimamoto K., Matsuda K., Masuda A., Takizawa H., Higashiura K., Miyazaki Y., Hirata A., Ura N., Nakagawa M. Effects of angiotensin receptor antagonist and angiotensin converting enzyme inhibitor on insulin sensitivity in fructose-fed hypertensive rats and essential hypertensives. Am J Hypertens. 1995;8(4 Pt 1):353–357. doi: 10.1016/0895-7061(94)00245-7. [DOI] [PubMed] [Google Scholar]
- 13.Julius S., Kjeldsen S.E., Weber M., Brunner H.R., Ekman S., Hansson L., Hua T., Laragh J., McInnes G.T., Mitchell L., Plat F., Schork A., Smith B., Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 14.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 15.Seferovic J.P., Claggett B., Seidelmann S.B., Seely E.W., Packer M., Zile M.R., Rouleau J.L., Swedberg K., Lefkowitz M., Shi V.C., Desai A.S., McMurray J.J.V., Solomon S.D. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M., Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21(8):63. doi: 10.1007/s11906-019-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa K., Wakino S., Simic P., Sakamaki Y., Minakuchi H., Fujimura K., Hosoya K., Komatsu M., Kaneko Y., Kanda T., Kubota E., Tokuyama H., Hayashi K., Guarente L., Itoh H. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umino H., Hasegawa K., Minakuchi H., Muraoka H., Kawaguchi T., Kanda T., Tokuyama H., Wakino S., Itoh H. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces Sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8(1):6791. doi: 10.1038/s41598-018-25054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Körner A., Eklöf A.C., Celsi G., Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43(5):629–633. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 20.Curthoys N.P., Moe O.W. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014;9(9):1627–1638. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rønnestad I., Akiba Y., Kaji I., Kaunitz J.D. Duodenal luminal nutrient sensing. Curr Opin Pharmacol. 2014;19:67–75. doi: 10.1016/j.coph.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki M., Akiba Y., Kaunitz J.D. Duodenal chemosensing. Curr Opin Gastroenterol. 2018;34(6):422–427. doi: 10.1097/MOG.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolskee R.F., Dyer J., Kokrashvili Z., Salmon K.S., Ilegems E., Daly K., Maillet E.L., Ninomiya Y., Mosinger B., Shirazi-Beechey S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsahli M., Gerich J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res Clin Pract. 2017;133:1–9. doi: 10.1016/j.diabres.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M., Johansen O.E., Woerle H.J., Broedl U.C., Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 28.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., Chertow G.M., Greene T., Hou F.F., Mann J.F.E., McMurray J.J.V., Lindberg M., Rossing P., Sjöström C.D., Toto R.D., Langkilde A.M., Wheeler D.C. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler D.C., Toto R.D., Stefansson B.V., Jongs N., Chertow G.M., Greene T., Hou F.F., McMurray J.J.V., Pecoits-Filho R., Correa-Rotter R., Rossing P., Sjostrom C.D., Umanath K., Langkilde A.M., Heerspink H.J.L., Committees D.-C.T. Investigators. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Benotti P.N., Wood G.C., Carey D.J., Mehra V.C., Mirshahi T., Lent M.R., Petrick A.T., Still C., Gerhard G.S., Hirsch A.G. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryuzaki M., Miyashita K., Sato M., Inoue H., Fujii K., Hagiwara A., et al. Activation of the intestinal tissue renin-angiotensin system by transient sodium loading in salt-sensitive rats. J Hypertens. 2022;40(1):33–45. doi: 10.1097/HJH.0000000000002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baud G., Daoudi M., Hubert T., Raverdy V., Pigeyre M., Hervieux E., Devienne M., Ghunaim M., Bonner C., Quenon A., Pigny P., Klein A., Kerr-Conte J., Gmyr V., Caiazzo R., Pattou F. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metabol. 2016;23(3):547–553. doi: 10.1016/j.cmet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Rahmoune H., Thompson P.W., Ward J.M., Smith C.D., Hong G., Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.X., Levi J., Luo Y., Myakala K., Herman-Edelstein M., Qiu L., Wang D., Peng Y., Grenz A., Lucia S., Dobrinskikh E., D'Agati V.D., Koepsell H., Kopp J.B., Rosenberg A.Z., Levi M. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, infiltration, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292(13):5335–5348. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata S., Mu S., Kawarazaki H., Muraoka K., Ishizawa K., Yoshida S., Kawarazaki W., Takeuchi M., Ayuzawa N., Miyoshi J., Takai Y., Ishikawa A., Shimosawa T., Ando K., Nagase M., Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121(8):3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata S., Nagase M., Yoshida S., Kawarazaki W., Kurihara H., Tanaka H., Miyoshi J., Takai Y., Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T., Kurihara I., Kobayashi S., Yokota K., Murai-Takeda A., Mitsuishi Y., Morisaki M., Kohata N., Oshima Y., Minami Y., Shibata H., Itoh H. Intestinal mineralocorticoid receptor contributes to epithelial sodium channel-mediated intestinal sodium absorption and blood pressure regulation. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.117.008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi T., Shibata H., Kurihara I., Yokota K., Mitsuishi Y., Ohashi K., Murai-Takeda A., Jo R., Ohyama T., Sakamoto M., Tojo K., Tajima N., Utsunomiya K., Itoh H. High glucose stimulates mineralocorticoid receptor transcriptional activity through the protein kinase C β-signaling. Int Heart J. 2017;58(5):794–802. doi: 10.1536/ihj.16-649. [DOI] [PubMed] [Google Scholar]
- 39.Yokota K., Shibata H., Kurihara I., Kobayashi S., Suda N., Murai-Takeda A., Saito I., Kitagawa H., Kato S., Saruta T., Itoh H. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem. 2007;282(3):1998–2010. doi: 10.1074/jbc.M607741200. [DOI] [PubMed] [Google Scholar]
- 40.Mitsuishi Y., Shibata H., Kurihara I., Kobayashi S., Yokota K., Murai-Takeda A., Hayashi T., Jo R., Nakamura T., Morisaki M., Itoh H. Epidermal growth factor receptor/extracellular signal-regulated kinase pathway enhances mineralocorticoid receptor transcriptional activity through protein stabilization. Mol Cell Endocrinol. 2018;473:89–99. doi: 10.1016/j.mce.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Shibata H., Itoh H. Mineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertension. Am J Hypertens. 2012;25(5):514–523. doi: 10.1038/ajh.2011.245. [DOI] [PubMed] [Google Scholar]
- 42.Aoi W., Niisato N., Sawabe Y., Miyazaki H., Tokuda S., Nishio K., Yoshikawa T., Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol Int. 2007;31(10):1288–1291. doi: 10.1016/j.cellbi.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Livne A., Balfe J.W., Veitch R., Marquez-Julio A., Grinstein S., Rothstein A. Increased platelet Na+-H+ exchange rates in essential hypertension: application of a novel test. Lancet. 1987;1(8532):533–536. doi: 10.1016/s0140-6736(87)90176-0. [DOI] [PubMed] [Google Scholar]
- 44.Feig P.U., D'Occhio M.A., Boylan J.W. Lymphocyte membrane sodium-proton exchange in spontaneously hypertensive rats. Hypertension. 1987;9(3):282–288. doi: 10.1161/01.hyp.9.3.282. [DOI] [PubMed] [Google Scholar]
- 45.Kuro-o M., Hanaoka K., Hiroi Y., Noguchi T., Fujimori Y., Takewaki S., Hayasaka M., Katoh H., Miyagishi A., Nagai R., et al. Salt-sensitive hypertension in transgenic mice overexpressing Na(+)-proton exchanger. Circ Res. 1995;76(1):148–153. doi: 10.1161/01.res.76.1.148. [DOI] [PubMed] [Google Scholar]
- 46.O'Hagan M., Howey J., Greene S.A. Increased proximal tubular reabsorption of sodium in childhood diabetes mellitus. Diabet Med. 1991;8(1):44–48. doi: 10.1111/j.1464-5491.1991.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 47.Bank N., Aynedjian H.S. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest. 1990;86(1):309–316. doi: 10.1172/JCI114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heck G.L., Mierson S., DeSimone J.A. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223(4634):403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 49.Chandrashekar J., Kuhn C., Oka Y., Yarmolinsky D.A., Hummler E., Ryba N.J., Zuker C.S. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464(7286):297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigiani A. Does ENaC work as sodium taste receptor in humans? Nutrients. 2020;12(4):1195. doi: 10.3390/nu12041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyata K.N., Lo C.S., Zhao S., Liao M.C., Pang Y., Chang S.Y., Peng J., Kretzler M., Filep J.G., Ingelfinger J.R., Zhang S.L., Chan J.S.D. Angiotensin II up-regulates sodium-glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice. Clin Sci (Lond) 2021;135(7):943–961. doi: 10.1042/CS20210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park S.H., Belcastro E., Hasan H., Matsushita K., Marchandot B., Abbas M., et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20(1):65. doi: 10.1186/s12933-021-01252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Oliveira T.L., Lincevicius G.S., Shimoura C.G., Simoes-Sato A.Y., Garcia M.L., C Tb R.R.C. Effects of renal denervation on cardiovascular, metabolic and renal functions in streptozotocin-induced diabetic rats. Life Sci. 2021;278:119534. doi: 10.1016/j.lfs.2021.119534. [DOI] [PubMed] [Google Scholar]
- 54.Gueguen C., Burke S.L., Barzel B., Eikelis N., Watson A.M.D., Jha J.C., Jackson K.L., Sata Y., Lim K., Lambert G.W., Jandeleit-Dahm K.A.M., Cooper M.E., Thomas M.C., Head G.A. Empagliflozin modulates renal sympathetic and heart rate baroreflexes in a rabbit model of diabetes. Diabetologia. 2020;63(7):1424–1434. doi: 10.1007/s00125-020-05145-0. [DOI] [PubMed] [Google Scholar]
- 55.Castoldi G., Carletti R., Ippolito S., Colzani M., Barzaghi F., Stella A., et al. Sodium-glucose cotransporter 2 inhibition prevents renal fibrosis in cyclosporine nephropathy. Acta Diabetol. 2021;58(8):1059–1070. doi: 10.1007/s00592-021-01681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawano Y., Nakae J., Watanabe N., Kikuchi T., Tateya S., Tamori Y., Kaneko M., Abe T., Onodera M., Itoh H. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metabol. 2016;24(2):295–310. doi: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Park J., Lee I.S., Kim K.H., Kim Y., An E.J., Jang H.J. GI inflammation increases sodium-glucose cotransporter Sglt1. Int J Mol Sci. 2019;20(10):2537. doi: 10.3390/ijms20102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V., Pedersen H.K., Arumugam M., Kristiansen K., Voigt A.Y., Vestergaard H., Hercog R., Costea P.I., Kultima J.R., Li J., Jørgensen T., Levenez F., Dore J., Nielsen H.B., Brunak S., Raes J., Hansen T., Wang J., Ehrlich S.D., Bork P., Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de la Cuesta-Zuluaga J., Mueller N.T., Corrales-Agudelo V., Velásquez-Mejía E.P., Carmona J.A., Abad J.M., Escobar J.S. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 60.Tappenden K.A., Drozdowski L.A., Thomson A.B., McBurney M.I. Short-chain fatty acid-supplemented total parenteral nutrition alters intestinal structure, glucose transporter 2 (GLUT2) mRNA and protein, and proglucagon mRNA abundance in normal rats. Am J Clin Nutr. 1998;68(1):118–125. doi: 10.1093/ajcn/68.1.118. [DOI] [PubMed] [Google Scholar]
- 61.Mishima E., Fukuda S., Kanemitsu Y., Saigusa D., Mukawa C., Asaji K., et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Ren Physiol. 2018;315(4):F824–F833. doi: 10.1152/ajprenal.00314.2017. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura N., Matsui T., Ishibashi Y., Yamagishi S. Insulin stimulates SGLT2-mediated tubular glucose absorption via oxidative stress generation. Diabetol Metab Syndrome. 2015;7:48. doi: 10.1186/s13098-015-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C., Zhang J., Xue M., Li X., Han F., Liu X., et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamezaki M., Kusaba T., Komaki K., Fushimura Y., Watanabe N., Ikeda K., Kitani T., Yamashita N., Uehara M., Kirita Y., Shiotsu Y., Sakai R., Fukuda T., Yamazaki M., Fukui M., Matoba S., Tamagaki K. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep. 2018;8(1):4029. doi: 10.1038/s41598-018-22229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swe M.T., Thongnak L., Jaikumkao K., Pongchaidecha A., Chatsudthipong V., Lungkaphin A. Dapagliflozin attenuates renal gluconeogenic enzyme expression in obese rats. J Endocrinol. 2020;245(2):193–205. doi: 10.1530/JOE-19-0480. [DOI] [PubMed] [Google Scholar]
- 66.Winiarska K., Jarzyna R., Dzik J.M., Jagielski A.K., Grabowski M., Nowosielska A., Focht D., Sierakowski B. ERK1/2 pathway is involved in renal gluconeogenesis inhibition under conditions of lowered NADPH oxidase activity. Free Radic Biol Med. 2015;81:13–21. doi: 10.1016/j.freeradbiomed.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Solini A., Rossi C., Mazzanti C.M., Proietti A., Koepsell H., Ferrannini E. Sodium-glucose co-transporter (SGLT)2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metabol. 2017;19(9):1289–1294. doi: 10.1111/dom.12970. [DOI] [PubMed] [Google Scholar]
- 68.Srinivasan Sridhar V., Ambinathan J.P.N., Kretzler M., Pyle L.L., Bjornstad P., Eddy S., Cherney D.Z., Reich H.N. European Renal c DNAB, Nephrotic Syndrome Study N. Renal SGLT mRNA expression in human health and disease: a study in two cohorts. Am J Physiol Ren Physiol. 2019;317(5):F1224–F1230. doi: 10.1152/ajprenal.00370.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajasekeran H., Reich H.N., Hladunewich M.A., Cattran D., Lovshin J.A., Lytvyn Y., Bjornstad P., Lai V., Tse J., Cham L., Majumder S., Bowskill B.B., Kabir M.G., Advani S.L., Gibson I.W., Sood M.M., Advani A., Cherney D.Z.I. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Ren Physiol. 2018;314(3):F412–F422. doi: 10.1152/ajprenal.00445.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moyad M.A. Review of potential risk factors for kidney (renal cell) cancer. Semin Urol Oncol. 2001;19(4):280–293. [PubMed] [Google Scholar]
- 71.Lindblad P., Chow W.H., Chan J., Bergström A., Wolk A., Gridley G., McLaughlin J.K., Nyrén O., Adami H.O. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42(1):107–112. doi: 10.1007/s001250051122. [DOI] [PubMed] [Google Scholar]
- 72.Labochka D., Moszczuk B., Kukwa W., Szczylik C., Czarnecka A.M. Mechanisms through which diabetes mellitus influences renal cell carcinoma development and treatment: a review of the literature. Int J Mol Med. 2016;38(6):1887–1894. doi: 10.3892/ijmm.2016.2776. [DOI] [PubMed] [Google Scholar]
- 73.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 74.Scafoglio C., Hirayama B.A., Kepe V., Liu J., Ghezzi C., Satyamurthy N., Moatamed N.A., Huang J., Koepsell H., Barrio J.R., Wright E.M. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–E4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuang H., Liao L., Chen H., Kang Q., Shu X., Wang Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Mon Int Med J Exp Clin Res. 2017;23:3737–3745. doi: 10.12659/MSM.902530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo G.F., Cai Y.C., Zhang B., Xu R.H., Qiu H.J., Xia L.P., Jiang W.Q., Hu P.L., Chen X.X., Zhou F.F., Wang F. Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis. Med Oncol. 2011;28(Suppl 1):S197–S203. doi: 10.1007/s12032-010-9696-8. [DOI] [PubMed] [Google Scholar]
- 77.Weihua Z., Tsan R., Huang W.C., Wu Q., Chiu C.H., Fidler I.J., Hung M.C. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13(5):385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Layton A.T., Vallon V., Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Ren Physiol. 2016;310(11):F1269–F1283. doi: 10.1152/ajprenal.00543.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor S.I., Blau J.E., Rother K.I. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849–2852. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a "thrifty substrate" hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 81.Kessler G., Friedman J. Metabolism of fatty acids and glucose. Circulation. 1998;98(13):1351. [PubMed] [Google Scholar]
- 82.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 83.Mudaliar S., Alloju S., Henry R.R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 84.Mizuno Y., Harada E., Nakagawa H., Morikawa Y., Shono M., Kugimiya F., Yoshimura M., Yasue H. The diabetic heart utilizes ketone bodies as an energy source. Metabolism. 2017;77:65–72. doi: 10.1016/j.metabol.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Tajima T., Yoshifuji A., Matsui A., Itoh T., Uchiyama K., Kanda T., Tokuyama H., Wakino S., Itoh H. β-hydroxybutyrate attenuates renal ischemia-reperfusion injury through its anti-pyroptotic effects. Kidney Int. 2019;95(5):1120–1137. doi: 10.1016/j.kint.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 86.Rojas-Morales P., Pedraza-Chaverri J., Tapia E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020;29:101395. doi: 10.1016/j.redox.2019.101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakraborty S., Galla S., Cheng X., Yeo J.Y., Mell B., Singh V., Yeoh B., Saha P., Mathew A.V., Vijay-Kumar M., Joe B. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25(3):677–689. doi: 10.1016/j.celrep.2018.09.058. e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liou A.P., Paziuk M., Luevano J.M., Jr., Machineni S., Turnbaugh P.J., Kaplan L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178) doi: 10.1126/scitranslmed.3005687. 178ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakatani H., Kasama K., Oshiro T., Watanabe M., Hirose H., Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Bastón J.I., Cid F.D., Caviedes-Vidal E., Chediack J.G. Daily expression of sodium-dependent glucose cotransporter-1 protein in jejunum during rat ontogeny. Anim Nutr. 2019;5(3):290–296. doi: 10.1016/j.aninu.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tavakkolizadeh A., Berger U.V., Shen K.R., Levitsky L.L., Zinner M.J., Hediger M.A., Ashley S.W., Whang E.E., Rhoads D.B. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G209–G215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 92.Solocinski K., Richards J., All S., Cheng K.Y., Khundmiri S.J., Gumz M.L. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Ren Physiol. 2015;309(11):F933–F942. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., Turek F.W., Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabol. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J.A., Ellisman M.H., Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabol. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]