Abstract
Background
With the growth in the popularity of facial filler injections, increased numbers of severe adverse events, such as cerebral embolism, have been reported.
Objectives
The aim of this article was to summarize the clinical manifestations and proposed mechanisms of filler-induced cerebral embolism (FICE).
Methods
A literature review was performed with the search keywords “filler injection,” “hyaluronic acid,” “fat graft,” “cerebral infarction,” “cerebral embolism,” “stroke,” “cerebrovascular infarction,” “disorders of consciousness,” and “hemiplegia.”
Results
Among the 43 cases of FICE enrolled from 35 articles, 37 patients were female, and 6 were male. Twenty-nine of these patients had received fat grafting, and 12 hyaluronic acid injection. Most FICE patients had been injected in the glabella, followed by the temporal, forehead, and nasal areas. Among 30 patients injected under local anesthesia, 43.33% presented with neurologic symptoms during the procedure. The main symptoms were consciousness disorders and hemiplegia. Most of the embolization sites were in the middle cerebral artery, followed by frontal lobe infarction and anterior cerebral artery infarction. Three patients developed cerebral hemorrhage after embolism. Twenty-six patients presented with newly acquired vision loss. The management for FICE cases included embolectomy, thrombolysis, decompressive craniectomy, antiplatelet/anticoagulant therapy, and symptomatic and nutritional treatment. Nearly half of the patients recovered or exhibited improved neurologic manifestations but not visual loss. Five patients died.
Conclusions
FICE is a severe complication following facial filler injection. Careful prevention, timely identification, and treatment are crucial to decreasing the morbidity and mortality of FICE.
Level of Evidence: 4

Facial contouring or volumization and the treatment of facial grooves, lines, depressions, or hollows can be achieved with synthetic off-the-shelf injectable facial fillers and autologous fat injections. Although the two techniques are conceptually and technically different, both are considered facial filler injections for the purposes of this review.
Injection of synthetic facial fillers is a widely used, minimally invasive facial cosmetic treatment that is increasing in popularity due to the ready availability, variety, ability to achieve natural outcomes, and perception of lower morbidity offered by these materials.1 FDA-approved absorbable/temporary materials used in these fillers include hyaluronic acid (HA), collagen, calcium hydroxyapatite, poly-l-lactic acid (PLA),2-4 and polymethylmethacrylate microspheres. The widely used term “dermal fillers” is actually a misnomer because most fillers can be placed at multiple levels of the soft tissue—subdermally, intramuscularly, or even deep on the periosteum. They should more correctly be called “soft tissue fillers.”
Autologous fat harvested by liposuction is also used as a facial filler although this is more correctly classified as a tissue-grafting procedure or operation. It is frequently performed worldwide and more so in Asia where facial 3-dimensional contouring is popular. Although autologous fat can be considered a filler injection, the method of insertion for fat grafts differs from that of synthetic filler injections: the latter does not require blood supply. Collectively, these injectable facial fillers (synthetic or fat) are an option in the treatment of age-related soft tissue volume loss, depressed scars, facial sculpting and contouring, augmentation of specific anatomic sites, wound reconstruction, and atrophy or asymmetry caused by disease.4
Synthetic facial filler injections are considered relatively safe with short recovery times and little risk of complications. General anesthesia or sedation is not required and most patients can return to work immediately. Mild and temporary adverse events such as swelling, bruising, redness, surface deformity, and infection can occasionally occur after synthetic facial filler injections1,4 and are acceptable risks. However, the increased use of synthetic facial injections has also led to a rise in reports of associated severe adverse events, such as hypersensitivity, cutaneous vascular complications with skin and tissue necrosis, blindness, and cerebral embolism.1,5,6
Facial fat injections, on the other hand, often require general anesthesia or intravenous sedation and are associated with higher rates of postoperative bruising, swelling, longer recovery times, and higher rates of morbidity that can include fat embolism and cerebral embolism.
Cerebral embolism associated with filler injections, whether by synthetic facial filler or facial fat injections, is a severe complication that has not received sufficient attention due to its low incidence rate and the lack of standardized approached to diagnosis, treatment, and prevention. The mechanism by which it occurs is not entirely clear and allows for healthy discussion. Here, we reviewed cerebral embolism cases induced by facial aesthetic filler injections, whether by synthetic fillers or fat grafting. We aimed to summarize the clinical manifestations, mechanisms, assessment, treatment, and prognosis of filler-induced cerebral embolism (FICE) patients, which may help clinicians understand this dreaded complication.
METHODS
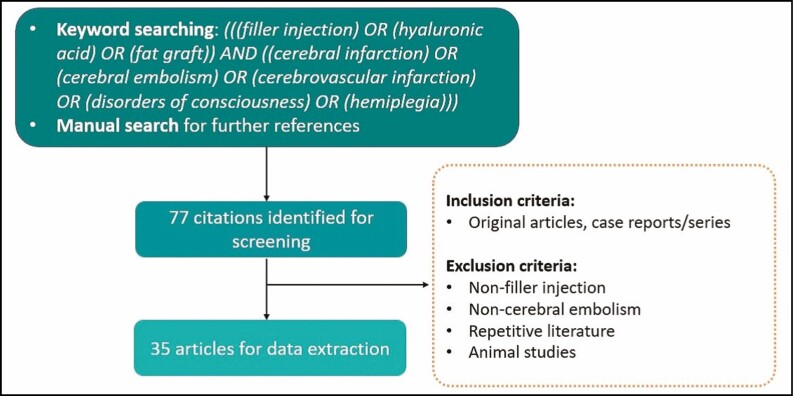
For this study, a literature review was performed in June 2020 (H.C.W. conducted the search and N.Y. reviewed it) according to the guidance provided by Murad et al (Figure 1).7 The following search terms were used in PubMed (United States National Library of Medicine [NLM], Bethesda, MD) to obtain all the relevant English-language literature published up to June 2020: “filler injection,” “hyaluronic acid,” “fat graft,” “cerebral infarction,” “cerebral embolism,” “stroke,” “cerebrovascular infarction,” “disorders of consciousness,” “hemiplegia.” The relevant articles selected for this study included original articles and case reports/series that investigated or discussed the role of filler injection in cerebral infarction. The articles excluded from this study were those utilizing non-filler injection or those discussing complications other than cerebral embolism. Articles presenting cerebral embolism as posters/abstracts were excluded, and animal studies were also excluded. The following data were extracted from the articles: author(s), year of publication, age and sex of patients, filler substance, injection site, anesthesia, symptoms and signs, onset time, diagnostic imaging results, infarction site, treatment, and prognosis. We were only able to analyze data captured in these articles, in some of which the reporting was incomplete.
Figure 1.
Flow diagram for study screening, selection, exclusion, and inclusion.
RESULTS
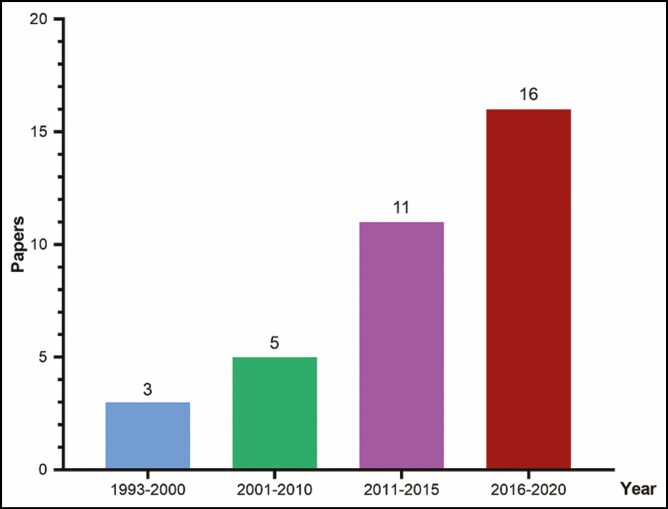
There were 35 articles reporting 43 cases of FICE in total enrolled for data extraction according to our inclusion and exclusion criteria (Table 1).5,6,8-40 Information about the publication year is shown in Figure 2.
Table 1.
The Data of the Enrolled Cases
| Year | Author | Age/sex | History | Anesthesia | Filler | Site | Neurologic signs (onset time) | Vision loss | Infarction site | Treatment | Prognosis (follow-up time) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | Bitar et al31 | 47/F | Healthy | Local | Fat | Glabella | Agnosia, facial paralysis, hemiplegia (during the operation) | Right eye | Frontal lobe, parietal lobe, MCA | Not known | Improved (3 weeks) |
| 1996 | Lee et al32 | 42/F | Not known | Local | Fat | Nasolabial fold | Headache, consciousness disorder (during the operation) | Left eye | Caudate lobe, thalamus, left cerebral hemisphere cortex | Hyperbaric oxygen therapy | Improved (3 months) |
| 1998 | Feinendegen et al33 | 47/F | Healthy | General | Fat | Nasolabial folds, lip, chin | Hemiplegia, aphasia, consciousness disorder (7 hours postoperation) | No | Frontal lobe, temporal lobe, MCA | Not known | Improved (4 months) |
| 2001 | Danesh et al34 | 43/M | Not known | Local | Fat | Nose, nasolabial fold | Headache, aphasia, hemiplegia (10 minutes postoperation) | Left eye | MCA | Not known | Not known |
| 2003 | Yoon et al35 | 39/F | Healthy | Local | Fat | Glabella | Aphasia, hemiplegia, consciousness disorder (1 minute postoperation) | Left eye | left hemisphere, ICA | Mechanical ventilation, steroids | Death |
| 2004 | Thaunat et al36 | 39/M | Left eye cancer | Local | Fat | Temporal, eyelids, glabella | Consciousness disorder (during the operation) | NA | ACA | Not known | Improved (1 year) |
| 2011 | Lee et al37 | 44/F | Not known | Intravenous | Fat | Periocular area | Dysarthria (2 hours postoperation) | Left eye | Insula, MCA | Mannitol, hyperbaric oxygen therapy | No improvement |
| 2010 | Lee et al38 | 24/F | Not known | Intravenous | Fat | Forehead | Motor disturbance, paresthesias (1 day postoperation) |
Left eye | MCA | Methylprednisolone | Improved (5 months) |
| 2010 | Toledano et al39 | 33/F | Eye wound | General | Fat | Left orbit | Hemiplegia (when awaking) | NA | MCA | Not known | Not known |
| 2011 | Hu et al40 | 28/F | Healthy | General | Fat | Temporal | Consciousness disorder, aphasia, hemiplegia (postoperation) | No | temporal lobe, parietal lobe, MCA | Mannitol, hydrocortisone, hyperbaric oxygen therapy, antiplatelet therapy | Improved (6 weeks) |
| 2012 | Park et al41 | 24/F | Healthy | Local | Fat | Glabella | Not known | Left eye | MCA | Not known | Not known |
| 26/F | Healthy | Local | Fat | Glabella | Not known | Left eye | MCA, ACA | Not known | Not known | ||
| 2013 | He et al42 | 52/F | Not known | Local | HA | Glabella | Headache (a few minutes postoperation) | Right eye | frontal lobe, occipital lobe, parietal lobe, ACA, MCA, PCA | Timolol maleate, acetazolamide, aspirin | Not known |
| 2014 | Hong et al10 | 27/F | Healthy | Local | Fat | Glabella, forehead, cheeks | Short-term memory disturbance, naming difficulty (several hours postoperation) | Left eye | frontal lobe | Not known | Improved mildly (1 year) |
| 50/F | Healthy | Local | HA | Glabella, cheeks | Dysarthria, hemiplegia, facial paralysis (24 hours postoperation) | Left eye | ACA, MCA, followed with cerebral hemorrhage at 2-week follow-up | Not known | Improved (6 months) | ||
| 2014 | Kim et al23 | 23/M | Not known | Local | HA | Nose | Right facial paralysis, left limb paralysis (during the operation) | Right eye | MCA, frontal, temporal and parietal lobes, followed by cerebral and subarachnoid hemorrhage by thrombolysis | Thrombolysis (plasminogen activator), decompressive craniectomy | No improvement (3 months) |
| 2014 | Kim et al43 | Not known/F | Healthy | Local | HA | Nose | Not known | Right eye | Frontal lobe | Corticosteroids | Not known |
| 2014 | Hong et al44 | 31/F | Not known | General | Fat | Glabella | Arm weakness (24 h postoperation) | Right eye | MCA, frontal lobe, parietal lobe, temporal lobe, occipital lobe | Not known | Recover (5 months) |
| 2014 | Wang et al45 | 22/F | Healthy | General | Fat | Forehead, temporal | Hemiplegia, Babinski sign (+), aphasia (5 hours postoperation) | Left eye | ACA, MCA, ICA, ECA | Decompressive craniectomy | Improved (2 months) |
| 2015 | Roshandel et al46 | 65/F | Hypertension | Not known | Fat | Forehead | Hemiplegia (several hours postoperation) | Right eye | frontal lobe, parietal lobe, occipital lobe, MCA | Not known | Not known |
| 2015 | Lin et al47 | 25/F | Healthy | Local | HA | Nose | Nausea, dizziness, weakness (4 hours postoperation) | Right eye | MCA | Not known | Not known |
| 2019 | Wang et al5 | 49/F | Healthy | Local | HA | Forehead | Consciousness disorder, headache, hemiplegia (during the operation) | No | Temporal, frontal and parietal lobes, followed with cerebral and subarachnoid hemorrhage | Low-molecular-weight heparin, clopidogrel, mannitol | Death |
| 2016 | Shen et al14 | 30/F | Healthy | Local | Fat | Temporal, chin | Consciousness disorder, left limb weakness, incontinence, vomiting (8 hours postoperation) | No | Right brain hemisphere, ICA, ECA, CCA, MCA, superficial temporal artery | Lowering intracranial pressure, antiplatelet aggregation, decompressive craniectomy | Improved (2 months) |
| 2016 | Kang et al48 | 32/F | Healthy | Local | Fat | Glabella | Consciousness disorder, aphasia, hemiplegia (during the operation) | Left eye | ACA, MCA | Thrombolytic agents | Improved (3 months) |
| 2016 | Li et al49 | 25/F | Healthy | Local | HA | Nose | Left upper limb weakness (9 hours postoperation) | Right eye | MCA | Not known | Not known |
| 2017 | Ragam et al19 | 55/F | Healthy | Local | PLA | Forehead | Dizziness, weakness, consciousness disorder (during the operation) | Right eye | ACA, frontal lobe, corpus callosum | Methylprednisolone | No improvement |
| 2018 | Marumo et al50 | 26/F | Healthy | Local | Hydroxyapatite | Glabella | Nausea, diplopia, consciousness disorder (during the operation) | Left eye | ECA | Not known | Not known |
| 2020 | Zhang et al24 | 31/F | Not known | Local | HA | Nose | Headache, nausea and vomiting, incontinence (5 minutes postoperation) | Left eye | MCA | Steroids, hyperbaric oxygen therapy, EHATSA | Improved |
| 46/F | Not known | Local | HA | Palpebra superior | Emotional disorder (hyperactivity) (not known) | Right eye | Lacunar cerebral infarction | Glucocorticoids, neurotrophic drug, hyperbaric oxygen, EHATSA | No improvement | ||
| 2020 | Liu et al51 | 35/F | Healthy | Not known | Fat | Not known | Hemiplegia (during the operation) | No | MCA, ECA | Aspirin, atorvastatin, dexamethasone | Recover (3 months) |
| 2020 | Yang et al6 | 40/F | Healthy | Local | HA | Nose | Nausea, vomiting, headache, consciousness disorders (30 minutes postoperation) | Left eye | Frontal lobe, parietal lobe, temporal lobe, occipital lobe | Mannitol, glucocorticoid, mechanical ventilation | Death |
| 2020 | Wang et al7 | 32/F | Healthy | Local | HA | Glabella | Emotional disorder (not known) | No | Frontal lobe | Antidepression therapy | Improved |
| 2019 | Zhou et al26 | 22/F | Healthy | Local | Fat | Temporal | Hemiplegia, consciousness disorder (4 hours postoperation) | No | ICA, MCA | Mechanical thrombectomy + thrombus aspiration technique | Improved (3 months) |
| 2019 | Ansari9 | 20/F | Healthy | Local | HA | Glabella | None | Right eye | parietal lobe, circle of Willis | Aspirin, prednisone | NA |
| 2019 | Liu et al17 | 42/F | Healthy | Local | Fat | Temporal | Lethargy, aphasia, hemiplegia (during the operation) | No | ICA, ACA, MCA, frontal, temporal and parietal lobes, superficial temporal artery | Decompressive craniectomy | No improvement (2 years) |
| 2019 | Renard et al52 | 50/M | Eye wound | General | Fat | Right orbit | Hemiplegia (when awaking) | NA | ACA, MCA | Not known | Not known |
| 2018 | Huo et al18 | 33/F | Healthy | Local | Fat | Glabella | Motor disturbance, consciousness disorder (during the operation) | No | ICA, MCA | Embolectomy + decompressive craniectomy | Improved |
| 25/F | Healthy | Local | Fat | Glabella | Motor disturbance, consciousness disorder (during the operation) | No | MCA, ACA, frontal, and temporal lobes | Embolectomy + decompressive craniectomy | Improved | ||
| 24/F | Healthy | Not known | Fat | Periocular | Seizure, consciousness disorder, motor disturbance (2 hours postoperation) | No | ACA, CCA, ICA, MCA, PCA | Embolectomy | Death | ||
| 19/M | Healthy | Not known | Fat | Glabella | Hemiplegia, consciousness disorder (1 hour postoperation) | No | MCA | Embolectomy + decompressive craniectomy | Improved | ||
| 28/M | Healthy | Not known | Fat | Glabella | Seizure, consciousness disorder (5 hours postoperation) | No | Not known | No | Death | ||
| 2018 | Wang et al16 | 22/F | Healthy | Local | Fat | Temporal | Consciousness disorder, limb weakness (during the operation) | Both | MCA, superficial temporal artery | Decompressive craniectomy | Improved (2 years) |
| 30/F | Healthy | Local | Fat | Temporal | Weakness (during the operation) | No | Right hemisphere, superficial temporal artery | Decompressive craniectomy | Not known |
ACA, anterior cerebral artery; CCA, common carotid artery; ECA, external carotid artery; EHATSA, endovascular hyaluronidase application through superselective angiography; ICA, internal carotid artery; MCA, middle cerebral artery; NA, not available; PCA, posterior cerebral artery.
Figure 2.
Publications reporting filler-induced cerebral embolism have increased over time.
Patients’ Characteristics
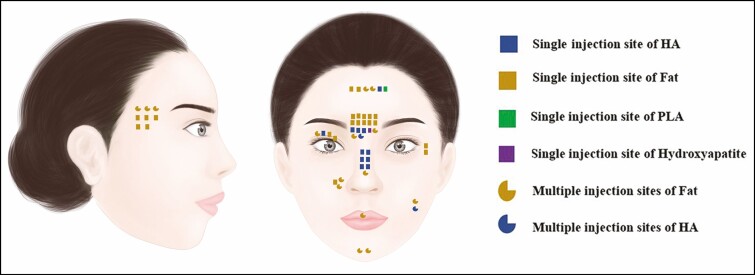
Among the 43 cases, 37 patients were female and 6 patients were male. The age of the patients ranged from 19 to 65 years (mean, 33.93 years). Twenty-nine (67.4%) patients received fat grafting, 12 (27.9%) patients received HA injection, 1 patient received PLA injection, and 1 patient received hydroxyapatite injection. Thirty-five (81.4%) patients had 1 injection site, 7 (16.3%) patients received injections at multiple sites, and 1 patient’s site was unknown. Of the 8 patients for whom the injection site was not specified, 6 (75%) received fat grafting. Data on the filler substance and injection sites are shown in Figure 3.
Figure 3.
Filler substances and injection sites. HA, hyaluronic acid; PLA, poly-l-lactic acid. Artwork created by author H. C. Wang, reproduced with permission from the author.
With regard to the clinical background of the patients, 30 (69.8%) patients were healthy, 3 patients had a history of eye conditions with visual loss due to cancer resection or trauma, 1 patient had hypertension, and the histories of 9 patients were unknown. Most of these cases were from East Asia.
Neurologic Manifestations
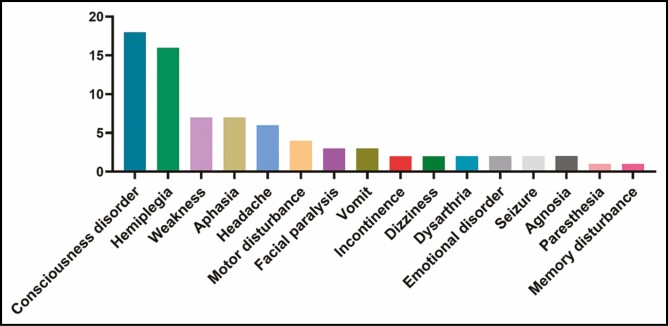
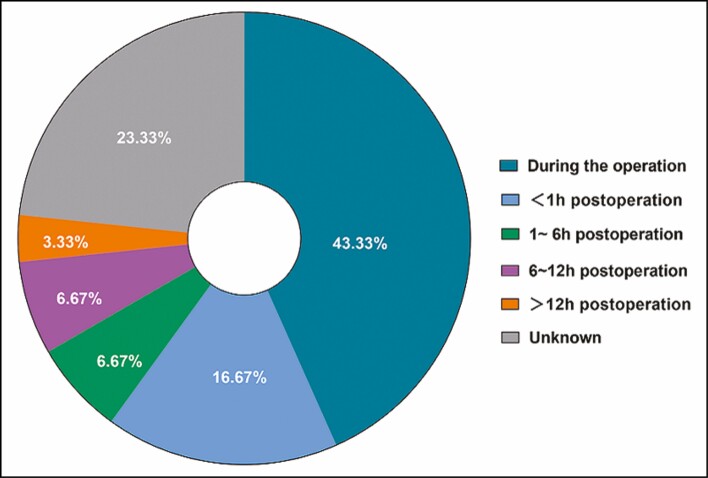
Regarding the neurologic manifestations (Figure 4), the main presenting symptoms were disorders of consciousness (n = 18) and hemiplegia (n = 16). Thirty (69.8%) patients received injections under local anesthesia, 6 (14.0%) patients received injections under general anesthesia, 2 patients received injections under intravenous sedation, and 5 patients’ anesthesia information was unknown. Among the 30 patients receiving local anesthesia, 43.33% presented with neurologic symptoms during the procedure, and 16.67% presented with similar symptoms within 2 hours postprocedure (Figure 5).
Figure 4.
Neurologic manifestations.
Figure 5.
The onset of neurologic manifestations in 30 patients receiving filler injections under local anesthesia.
Cerebral Embolism
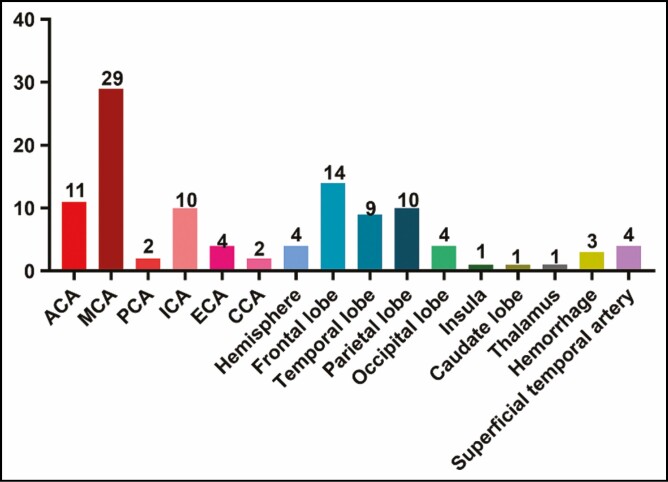
To confirm a diagnosis of cerebral embolism, 26 patients underwent a magnetic resonance imaging scan, 12 patients underwent diffusion-weighted imaging, 8 patients underwent a computed tomography scan, 5 patients underwent computed tomography angiography, and 2 cases underwent superselective cerebrovascular angiography. Most of the embolization sites were at the middle cerebral artery (n = 29), followed by frontal lobe infarction (n = 14) and anterior cerebral artery infarction (n = 11) (Figure 6). Notably, 3 patients developed cerebral hemorrhage after embolism.
Figure 6.
Embolization sites. ACA, anterior cerebral artery; CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
Vision Loss
Three patients had pre-existing visual loss (1 due to cancer resection, 2 due to orbital trauma). Of the 43 patients who suffered a FICE, 26 patients (60.5%) presented with concomitant vision loss and a stroke, whereas 17 patients (39.5%) presented only with FICE without any concomitant vision loss.
Among the 26 cases with vision loss, 5 patients receiving general anesthesia presented with vision loss after awakening, along with other neurologic signs. The remaining 21 patients had their procedures performed under local anesthesia: 10 of these patients (47.6%) presented with blindness and neurologic signs synchronously during or shortly postoperation (up to 2 hours). In 8 (23.8%) patients blindness appeared quickly postoperation, but their neurologic symptoms only occurred after several hours (at 4, 5, 8, 9, 24, 24, and 24 hours, respectively, and 1 case described as after “several hours”). The situation of 5 patients was unknown.
Among the 17 patients without concomitant vision loss, 5 patients (29.4%) had injections in the temporal area, 5 patients (29.4%) had injections in the glabella, 1 patient had injections in the forehead, 1 patient had an injection in the periocular region, 1 patient had an injection in the nasolabial folds, and 1 patient’s injection site was unknown.
Treatment and Prognosis
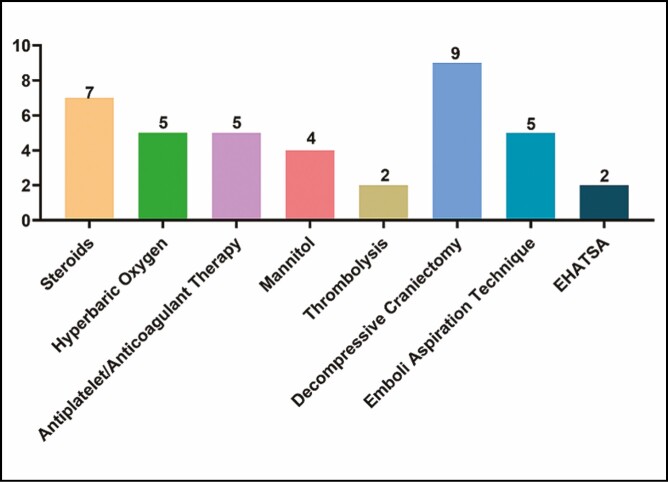
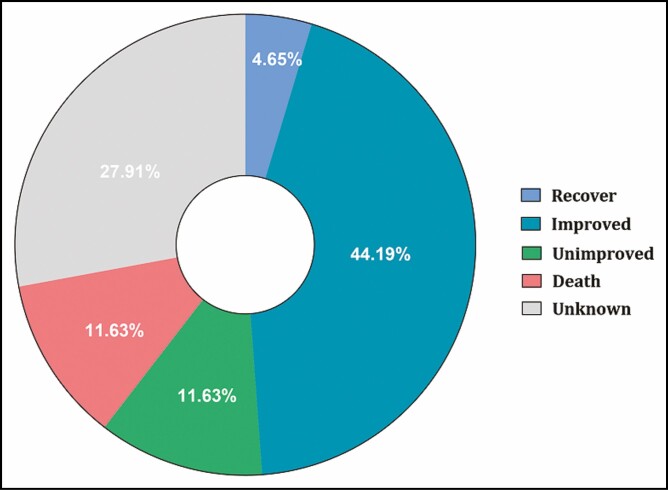
The treatment of 28 patients was described in the literature (Figure 7). Seven patients underwent embolectomy and 2 patients underwent thrombolysis. Nine patients received decompressive craniectomy. Five patients underwent antiplatelet/anticoagulant therapy. The rest received only symptomatic treatment and nutritional treatment, including steroids, nutritional neuropharmaceuticals, mannitol, and hyperbaric oxygen. Regarding the prognosis, 21 (48.8%) patients recovered or improved neurologically (Figure 8). Five patients (11.6%) remained unimproved and 5 (11.6%) patients died.
Figure 7.
Filler-induced cerebral embolism treatment. EHATSA, endovascular hyaluronidase application through superselective angiography.
Figure 8.
The prognosis of the filler-induced cerebral embolism patients.
DISCUSSION
Patients’ Characteristics
The patients with FICE were predominantly females. Moreover, the vast majority of these patients were from East Asia (only 9 out of 35 papers were from the West). This gender and ethnic bias may reflect the aesthetic aspirations of many Asian women today who wish to achieve an oval or heart-shaped face with smooth, full, and convex forehead contours and an absence of temporal hollows.41 This aesthetic trend has led to an increase in the use of autologous fat or synthetic fillers to create the desired convex shape. The forehead, brow, and temples are well-established danger zones where numerous arterial connections between the internal and external carotid artery systems exist as well as terminal arterial branches of the ophthalmic artery. These can be inadvertently punctured, leading to a filler embolus entering the internal carotid artery system, and may explain why so many patients with FICE are in fact Asian and women. Males, whether Asian or Caucasian, are less interested in shaping their foreheads and temporal regions, which may explain their lower risk for this severe adverse event.
Clinical Manifestations
The clinical manifestations of FICE were mainly neurologic symptoms and signs related to the location and degree of the obstruction, including hemiplegia and consciousness disorders. In the literature, the symptoms of some patients with mild obstruction were less recognizable, and were identified as obstruction by further imaging examinations. These patients sometimes presented with neuropsychiatric symptoms only, such as emotional or mood changes, memory disturbance, or asymptomatic lacunar infarction, which was seen only on imagings.8,13,37,39 Therefore, we speculate that the incidence of FICE may be much higher than that reported in the literature. We also noticed that the recognition of the onset of cerebral embolism symptoms was affected or delayed by the type of anesthesia used.
Generally, neurologic symptoms occurred during the operation or within 1 hour postoperation for the FICE patients receiving filler injection under local anesthesia. However, it took more time for the patients under general/intravenous anesthesia to complain about neurologic symptoms because they needed time to wake up from anesthesia. This may delay the timely diagnosis of cerebral embolism. Therefore, to detect cerebral embolism in time, patients recovering from general/intravenous anesthesia should be closely examined for neurologic signs, including muscle strength, muscle tension, pupillary light reflection, and pathologic reflexes.
In addition, due to the fact that a considerable number of patients with cerebral embolism were associated with immediate blindness, or even delayed blindness, which in turn was related to the mechanism of emboli entering the internal carotid or ophthalmic blood vessels at different times, those patients who complain only of ocular symptoms should also be carefully evaluated for signs and symptoms of cerebral embolism as well. We have noted 8 cases where the neurologic signs developed between 4 and 24 hours postoperatively.
Mechanism
In the literature the most frequent injection site associated with FICE was the glabella, followed by the temporal, forehead, and nasal areas. At present, it is thought that FICE and blindness is a direct result of accidental injection of filler material into a facial vessel that is a terminal branch of the ophthalmic artery (internal carotid artery system) or into a branch of the facial artery (external carotid artery system) that, in turn, anastamoses with branches of the internal carotid artery system. This leads to subsequent retrograde embolism and obstruction of certain cerebral vessels or the ophthalmic artery branches.
By analyzing the imaging findings of the FICE patients reported in the literature, we summarized the possible ways of the filler leading to intracranial vascular embolism into 3 types, which are described in Table 2.
Table 2.
Three Possible Ways for Filler to Lead to Intracranial Vascular Embolism
| Type | Mechanism | Route by which emboli enter the brain | Onset of neurologic signs | Other possible manifestations |
|---|---|---|---|---|
| I | The filler emboli enter the extracranial branches of the ophthalmic artery injured during the injection, or the anastomotic branches of the internal carotid artery and the external carotid artery injured during the injection | Extracranial branches of ophthalmic artery/anastomotic branches of ICA and ECA → ophthalmic artery → MCA | Immediate | Blindness |
| II | The filler emboli enter the superficial temporal artery, the filler emboli may be pushed into the external carotid artery or even the common carotid artery under excessive injection pressure | Superficial temporal artery → ECA → CCA → ICA → MCA | Immediate | Blindness |
| III | The filler emboli enter veins damaged during the injection | Veins on face → anterograde venous system → heart → brain/lung/eye | Delay | Delayed blindness, pulmonary embolism |
CCA, common carotid artery; ECA, external cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery.
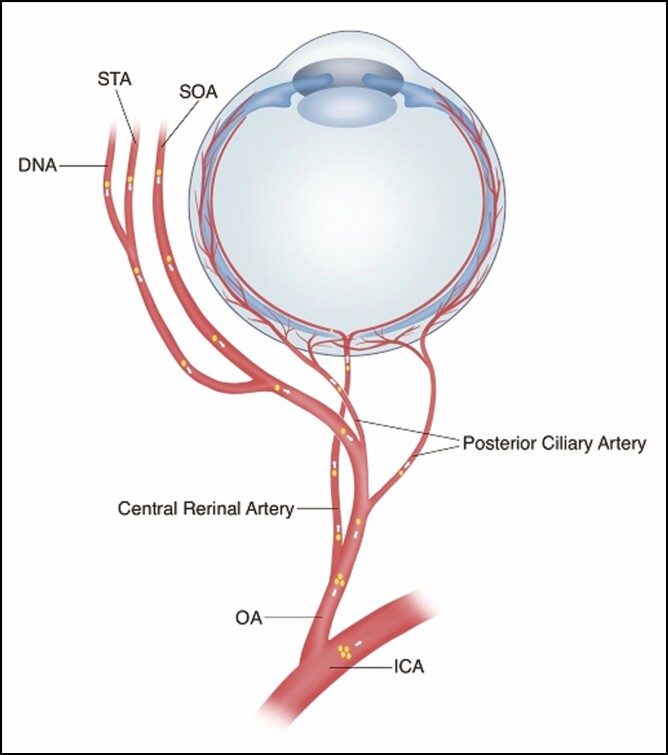
One possible mechanism is that during the injection procedure, the filler material was inadvertently injected under pressure into one of the extracranial terminal branches of the ophthalmic artery (supratrochlear, supraorbital, dorsal nasal, anterior ethmoidal, and lacrimal arteries) or injected into any of the anastomotic branches between the internal carotid and external carotid artery systems (Figure 9). Filler emboli may then reach the ophthalmic artery and/or the internal carotid artery. Blindness occurred when the central retinal artery (CRA), or posterior ciliary artery (PCA) was obstructed, whereas cerebral embolism occurred when the terminal intracranial branches of the internal carotid artery were obstructed (Figure 10).
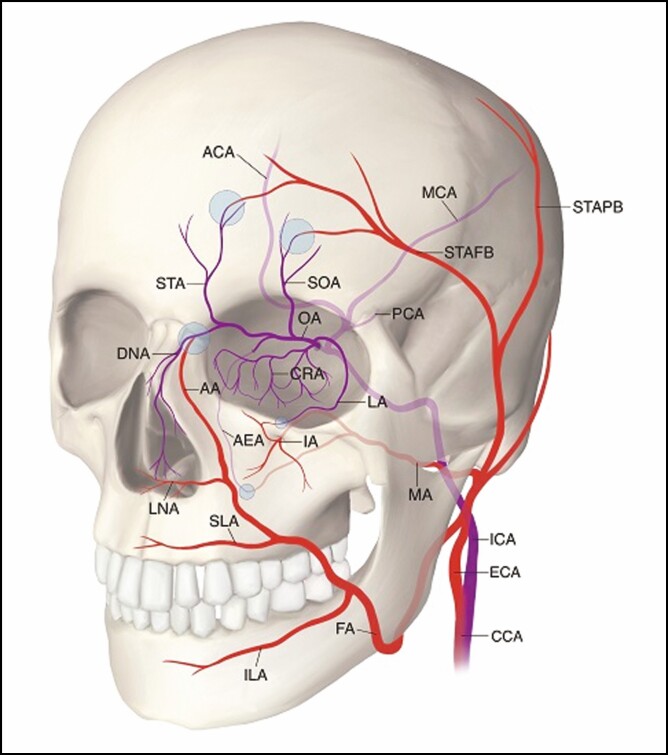
Figure 9.
Schematic diagram showing the extracranial branches of the ophthalmic artery and the anastomotic branches (purple circle) of the internal carotid artery (blue) and the external carotid artery (red). AA, angular artery; ACA, anterior cerebral artery; AEA, anterior ethmoidal artery; CCA, common carotid artery; CRA, central retinal artery; DNA, dorsal nasal artery; ECA, external carotid artery; FA, facial artery; IA, infraorbital artery; ICA, internal carotid artery; ILA, inferior labial artery; LA, lacrimal artery; LNA, lateral nasal artery; MA, maxillary artery; MCA, middle cerebral artery; OA, ophthalmic artery; PCA, posterior cerebral artery; SLA, superior labial artery; SOA, supraorbital artery; STA, supratrochlear artery; STAFB, superficial temporal artery frontal branch; STAPB, superficial temporal artery parietal branch. Artwork created by author R. Dong, reproduced with permission from the author.
Figure 10.
Schematic diagram showing the relation between blindness and cerebral embolism. CRA, central retinal artery; DNA, dorsal nasal artery; ICA, internal carotid artery; OA, ophthalmic artery; SOA, supraorbital artery; STA, supratrochlear artery. Artwork created by author R. Dong, reproduced with permission from the author.
It is a known anatomic fact that many anastomotic branches exist between the external and the internal carotid artery systems, and although there is significant human variation, 4 main groups of these anastomotic vessels are described:42
(1) The frontal (anterior) branch of the superficial temporal artery anastomoses with the lacrimal, palpebral, or supraorbital branches of the ophthalmic artery.
(2) The angular artery is a terminal branch of the facial artery, which is in turn derived from the external maxillary artery (external carotid artery system). The angular artery can anastomose directly with the inferior palpebral artery, the dorsal nasal artery, or the supratrochlear artery, all of which are terminal branches of the ophthalmic artery (internal carotid artery system). The supratrochlear or anterior ethmoidal arteries may continue down the dorsum of the nose as the dorsal nasal artery, which often anastomoses with the alar and sidewall branches of the facial artery.43
(3) The orbital branches of the middle meningeal pass through the superior orbital fissure, and anastomose with the lacrimal or other branches of the ophthalmic artery.
(4) The infraorbital branch of the internal maxillary artery may anastomose with the dorsal nasal branch of the ophthalmic artery.
It must be appreciated that there are many arterial and anastamotic variations, with some individuals having one and not the other, or even having multiple of these connections.
Whichever of these direct or anastamotic routes is traversed, it is reasonable to assume that a column of filler material must first find its way into either the dorsonasal, supratrochlear, or supraorbital arteries (all being externalized terminal branches of the ophthalmic artery) before it is forced retrogradely into the ophthalmic artery itself, past the origins of the central retinal and posterior ciliary arteries44 and subsequently past the origin of the ophthalmic artery from the internal carotid artery.
If the column of filler material is sufficient to go past where the CRA and PCA originate from (Figure 10) but not into the internal carotid artery, then that embolus may be carried anterogradely down the CRA or PCA and give rise to visual disturbances. A cerebral embolism would not occur in this situation.
If, however, the filler material traverses the entire length of the ophthalmic artery and enters the internal carotid artery, it can then potentially be carried anterogradely downstream until it reaches the middle cerebral artery or other intracranial vessels that derive from the internal carotid artery, and then eventually obstructs a terminal branch in the brain.
In Table 2, this is referred to as the Type I mechanism for cerebral embolism. This type of vascular embolism can cause immediate cerebral embolism and/or blindness. It has been shown in experimental and anatomic models that as little as 0.08 mL of HA filler can cause blindness.44 It is not known how much filler is required to induce a cerebral embolism.
In the Type II mechanism of cerebral embolism, the superficial temporal artery is injured during temporal injections. After entering the superficial temporal artery, the filler emboli may be pushed into the external carotid artery or even the common carotid artery under excessive injection pressure, which then flows into the internal carotid artery and the intracranial blood vessels, resulting in cerebral embolism and/or blindness.25,32,36,45 This kind of embolization can be preliminarily confirmed by loss of pulsation of the superficial temporal artery on palpation.16
The Type III mechanism occurs when the veins of the face are damaged during the injection process. The filler then embolizes to the heart first, after which it follows the systemic circulation, before it reaches the terminal branches and causes vascular complications, such as pulmonary embolism, cerebral embolism, or blindness.29,46-48 The manifestations of this type of vascular embolism may present hours after the initial injection; 29,46 multiple factors, such as overinjection, injection displacement, muscle activity, and other factors, may also play a role in vascular complications of filler injection.
Cannula vs Sharp Needle
The debate over which one of these modalities is safer continues. It was initially thought that a blunt cannula would be a safer instrument than a sharp needle for facial filler injections. However, both cannulas and sharp needles have been seen to penetrate blood vessels and cause filler emboli.
A more important consideration would be at which level of soft tissue (subdermal, superficial fat, fascia, muscle, deep fat, or periosteum) these fillers were injected and whether there are major blood vessels in the vicinity that could potentially be penetrated by either of these instruments.
The 35 articles surveyed for this systematic review are silent on the use of cannulas but it can safely be assumed that in all the patients who received facial fat injections (29 out of 43, 67.4%) the injections would have been performed with a cannula, as is the usual practice. Of the 12 patients who received HA filler injections (27.9%), we can also extrapolate that at least 50% of these would have been administered by a cannula. This implies that up to 82% of all FICE cases may have been associated with the use of a cannula.
This alarming statistic could be due to the very nature of facial fat injections as they require the fat to be delivered in multiple passes and evenly distributed throughout the middle lamellar (muscle) of the soft tissue so as to allow a blood supply to develop. In the face, the arteries lie mainly in or just deep to the middle lamella of facial muscles, and therefore multiple passes of the cannula in this layer increase the risk of vascular damage and penetration.
With regard to the depth of injection, detailed knowledge of facial anatomy would suggest that the subdermal and/or supraperiosteal layers are safer for injection because there are few major arteries running in these 2 layers. In our opinion, it makes sense to inject immediately subdermally in the subcutaneous fat or deep on the bone (avoiding the vascular bony foramina such as the infraorbital or supraorbital foramen) to reduce inadvertent penetration of the blood vessels (such as the facial or superficial temporal arteries) which are more intimately associated with the layer of facial muscles (the middle lamella). Injecting into either the subdermal or preperiosteal layers is possibly easier to achieve with a sharp needle than a cannula. A cannula may provide the injector with a false sense of security, leading to more vascular trauma.
Treatment
The current treatment of FICE includes general symptomatic and nutritional therapies such as hyperbaric oxygen, neuropharmaceuticals, mannitol, and steroids. Thrombolytic therapy or antiplatelet/anticoagulant therapy is not only ineffective for patients with cerebral embolism induced by filler injection (which is a nonthrombotic embolism), it may also cause drug-induced cerebral hemorrhage, aggravating the patient’s condition.5,19 For vascular embolism caused by HA, some researchers have tried to achieve recanalization of the occluded vessel by administering intra-arterial thrombolysis therapy with the injection of hyaluronidase and/or urokinase. In a study of 24 patients with vision loss caused by HA injection, 42% of the patients ultimately showed improvements in visual acuity following intra-arterial thrombolysis therapy even when the recommended window for optimal thrombolytic treatment had passed.49 In addition, the authors found that hyaluronidase combined with urokinase was a more effective therapy than hyaluronidase alone. In cases of filler-induced blindness, we know that extravascular or retrobulbar hyaluronidase plays little or no role in resolving visual loss.44,50 For embolization due to fat, some researchers have used an emboli aspiration technique to partially recanalize the occluded vessel; however, this technique carries the potential risk of triggering distant cerebrovascular embolism.16,40
The prognosis of FICE is related to which vessels are occluded. Unlike the obstruction of small blood vessels leading to mild symptoms with a good prognosis, occlusion of large blood vessels can lead to a large-area cerebral infarction with secondary exacerbation of the edema, which was associated with high morbidity and high mortality.18 Under such circumstances, decompression craniectomy is strongly recommended to reduce mortality.16 It is crucial for FICE patients to be diagnosed and treated in time. Delayed treatment may also lead to poor prognosis.16,40
Prevention
With treatment options for FICE being somewhat limited, and to an extent ineffective, it would be prudent for practitioners around the world to be acutely aware of this complication and take the necessary steps to reduce its occurrence. From the papers sourced for this review, we notice a worrying trend in the occurrence of FICE. Between 1993 and 2010 (17 years) 8 cases of FICE were reported. This jumped to 11 cases between 2011 and 2015 (4 years) and a further jump to 16 cases in the period 2016 to 2020 (4 years). This means that in the last 8 years the total number of FICE cases has risen to 27, more than 3.5 times that in the period 1993 to 2010 (Figure 2).
Most of these cases were from East Asia and the majority were associated with facial fat injections with cannulas. This alarming increase in the frequency of FICE occurrence may reflect the current situation where there is a proliferation of underground illegal cosmetic clinics in this region. Here, untrained operators without a semblance of understanding of anatomy are more likely to damage blood vessels when injecting filler.
For medical practitioners performing these procedures, some measures have been widely recommended to reduce the incidence of vascular complications during the injection process. These include: (1) aspirating and maintaining negative pressure upon entry all the way to the designated site; (2) injecting small volumes slowly while closely observing the injection site and the patient’s reaction—physicians should avoid using too many fillers at one time; (3) injecting filler with blunt cannulas rather than needles. This last point is arguable because it flies in the face of the evidence we have—over 80% of FICE patients in this review were likely treated with cannula injections. This therefore contradicts the dictum that “cannulas are safer.” In fact, delivering filler directly to the bone with a sharp needle may provide a safer alternative than cannulas which may give the injector a false sense of security.
We submit that, for injecting fillers, the critical skill a medical practitioner should possess is a sound knowledge of facial anatomy—so that they know the course and changing levels of the major vessels, have an understanding of the sites of anastomosis between internal and carotid artery systems, and are familiar with the different soft tissue layers and their relations with the arteries of the face.51
Even then, these measures may still fail to completely avoid the occurrence of filler-associated vascular complications, due to multiple factors such as anatomic variations and operator skill. The development of ultrasound-assisted injection may allow practitioners to clearly recognize the location of blood vessels in relation to the different anatomic layers52-54 of soft tissue. The use of ultrasound-assisted injections, although requiring training, is not that complicated to master. This may be the trend for filler injection methods in the future. We also recommend performing filler injections under local anesthesia as much as possible in order to identify and treat FICE in a timely manner.
CONCLUSIONS
FICE is a severe complication following facial filler injection with limited treatment options. Careful prevention and timely identification and treatment are crucial to decrease the morbidity and mortality of FICE.
Contributor Information
Hayson Chenyu Wang, Division of Plastic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Nanze Yu, Division of Plastic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xiaojun Wang, Division of Plastic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Ruijia Dong, Division of Plastic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xiao Long, Division of Plastic Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xin Feng, Department of Neurosurgery, Beijing Hospital, Beijing, China.
Jianle Li, Department of Neurology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Dr Hayson Chenyu Wang was supported by the China Scholarship Council (201906210435).
REFERENCES
- 1. Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: a systematic review of literature. Aesthetic Plast Surg. 2020;44(3):929-944. [DOI] [PubMed] [Google Scholar]
- 2. Massone C, Horn M, Kerl H, Ambros-Rudolph CM, Giovanna Brunasso AM, Cerroni L. Foreign body granuloma due to Matridex injection for cosmetic purposes. Am J Dermatopathol. 2009;31(2):197-199. [DOI] [PubMed] [Google Scholar]
- 3. Grippaudo FR, Mattei M. High-frequency sonography of temporary and permanent dermal fillers. Skin Res Technol. 2010;16(3):265-269. [DOI] [PubMed] [Google Scholar]
- 4. Wang C, Sun T, Li H, Li Z, Wang X. Hypersensitivity caused by cosmetic injection: systematic review and case report. Aesthetic Plast Surg. 2021;45(1):263-272. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Wang X. Cerebral hemorrhage after cosmetic facial injection. Plast Reconstr Surg Glob Open. 2019;7(9):e2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Q, Lu B, Guo N, et al. Fatal cerebral infarction and ophthalmic artery occlusion after nasal augmentation with hyaluronic acid—a case report and review of literature. Aesthetic Plast Surg. 2020;44(2):543-548. [DOI] [PubMed] [Google Scholar]
- 7. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ansari ZA, Choi CJ, Rong AJ, Erickson BP, Tse DT. Ocular and cerebral infarction from periocular filler injection. Orbit. 2019;38(4):322-324. [DOI] [PubMed] [Google Scholar]
- 9. Bitar S, Gomez CR. Stroke following injection of a melted suppository. Stroke. 1993;24(5):741-743. [DOI] [PubMed] [Google Scholar]
- 10. Danesh-Meyer HV, Savino PJ, Sergott RC. Case reports and small case series: ocular and cerebral ischemia following facial injection of autologous fat. Arch Ophthalmol. 2001;119(5):777-778. [PubMed] [Google Scholar]
- 11. Feinendegen DL, Baumgartner RW, Vuadens P, et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163-167. [DOI] [PubMed] [Google Scholar]
- 12. He MS, Sheu MM, Huang ZL, Tsai CH, Tsai RK. Sudden bilateral vision loss and brain infarction following cosmetic hyaluronic acid injection. JAMA Ophthalmol. 2013;131(9):1234-1235. [DOI] [PubMed] [Google Scholar]
- 13. Hong JH, Ahn SJ, Woo SJ, et al. Central retinal artery occlusion with concomitant ipsilateral cerebral infarction after cosmetic facial injections. J Neurol Sci. 2014;346(1- 2): 310-314. [DOI] [PubMed] [Google Scholar]
- 14. Hong DK, Seo YJ, Lee JH, Im M. Sudden visual loss and multiple cerebral infarction after autologous fat injection into the glabella. Dermatol Surg. 2014;40(4):485-487. [DOI] [PubMed] [Google Scholar]
- 15. Hu J, Chen W, Wu Y, et al. Middle cerebral artery occlusion following autologous bitemporal fat injection. Neurol India. 2011;59(3):474-475. [DOI] [PubMed] [Google Scholar]
- 16. Huo X, Liu R, Wang Y, et al. Cerebral fat embolism as complication of facial fat graft: retrospective analysis of clinical characteristics, treatment, and prognosis. World Neurosurg. 2018;120:249-255. [DOI] [PubMed] [Google Scholar]
- 17. Kang JH, Park KH, Park JS. Acute mental change and hemiplegia after autologous fat injection. J Cosmet Laser Ther. 2016;18(7):413-416. [DOI] [PubMed] [Google Scholar]
- 18. Kim SN, Byun DS, Park JH, et al. Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci. 2014;21(4):678-680. [DOI] [PubMed] [Google Scholar]
- 19. Kim EG, Eom TK, Kang SJ. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel. J Craniofac Surg. 2014;25(2):684-686. [DOI] [PubMed] [Google Scholar]
- 20. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YJ, Kim HJ, Choi KD, Choi HY. MRI restricted diffusion in optic nerve infarction after autologous fat transplantation. J Neuroophthalmol. 2010;30(3):216-218. [DOI] [PubMed] [Google Scholar]
- 22. Lee CM, Hong IH, Park SP. Ophthalmic artery obstruction and cerebral infarction following periocular injection of autologous fat. Korean J Ophthalmol. 2011;25(5):358-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li KT, Huang YH, Chen CH, Chou LW. Delayed-onset cerebral infarction after cosmetic facial injection using hyaluronic acid. J Formos Med Assoc. 2016;115(7):587-588. [DOI] [PubMed] [Google Scholar]
- 24. Lin YC, Chen WC, Liao WC, Hsia TC. Central retinal artery occlusion and brain infarctions after nasal filler injection. QJM 2015;108(9):731-732. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Wu X, Zhang X, Niu C, Zhu H. Internal carotid artery embolism after autologous fat injection for temporal augmentation. Aesthetic Plast Surg. 2019;43(2):383-387. [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Yin M, Liu S, Hu M, Zhang B. Facial filler causes stroke after development of cerebral fat embolism. Lancet. 2020;395(10222):449. [DOI] [PubMed] [Google Scholar]
- 27. Marumo Y, Hiraoka M, Hashimoto M, Ohguro H. Visual impairment by multiple vascular embolization with hydroxyapatite particles. Orbit. 2018;37(3):165-170. [DOI] [PubMed] [Google Scholar]
- 28. Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154(4):653-62 e1. [DOI] [PubMed] [Google Scholar]
- 29. Ragam A, Agemy SA, Dave SB, Khorsandi AS, Banik R. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37(1):77-80. [DOI] [PubMed] [Google Scholar]
- 30. Renard D, Charavel P, Dahmani L, Freitag C. Cerebral fat embolism after autologous fat injection for reconstructive eye surgery. Rev Neurol (Paris). 2019;175(1- 2): 94-95. [DOI] [PubMed] [Google Scholar]
- 31. Roshandel D, Soheilian M, Pakravan M, Aghayan S, Peyman GA. Middle cerebral artery, ophthalmic artery, and multibranch retinal vessel occlusion after cosmetic autologous fat transfer to forehead. Ophthalmic Surg Lasers Imaging Retina. 2015;46(5):593-596. [DOI] [PubMed] [Google Scholar]
- 32. Shen X, Li Q, Zhang H. Massive cerebral infarction following facial fat injection. Aesthetic Plast Surg. 2016;40(5):801-805. [DOI] [PubMed] [Google Scholar]
- 33. Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat embolism induced by facial fat injection. Plast Reconstr Surg. 2004;113(7):2235-2236. [DOI] [PubMed] [Google Scholar]
- 34. Toledano S, Zyss J, Gerber S, Rodallec M, Zuber M. Fat emboli responsible for ischemic stroke in reconstructive eye surgery. J Neurol. 2010;257(11):1927-1928. [DOI] [PubMed] [Google Scholar]
- 35. Wang DW, Yin YM, Yao YM. Internal and external carotid artery embolism following facial injection of autologous fat. Aesthet Surg J. 2014;34(8):NP83-NP87. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Wu M, Zhou X, Liu H, Zhang Y, Wang H. Autologous fat used for facial filling can lead to massive cerebral infarction through middle cerebral artery or facial intracranial branches. J Craniofac Surg. 2018;29(5):1341-1343. [DOI] [PubMed] [Google Scholar]
- 37. Wang C, Sun T, Zhu L, Zhang Y, Wang X. Emotional disorder syndrome after cosmetic facial injection. J Cosmet Dermatol. 2020;19(9):2273-2276. [DOI] [PubMed] [Google Scholar]
- 38. Yoon SS, Chang DI, Chung KC. Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61(8):1151-1152. [DOI] [PubMed] [Google Scholar]
- 39. Zhang L, Luo Z, Li J, et al. Endovascular hyaluronidase application through superselective angiography to rescue blindness caused by hyaluronic acid injection. Aesthet Surg J. 2021;41(3):344-355. [DOI] [PubMed] [Google Scholar]
- 40. Zhou K, Cai C. The successful mechanical lipectomy treatment of cerebral fat embolism following autologous fat injection. Plast Reconstr Surg Glob Open. 2019;7(1):e2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu WT, Liew S, Chan HH, et al. Consensus on current injectable treatment strategies in the Asian face. Aesthetic Plast Surg 2016;40(2):202-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taveras JM, Mount LA, Friedenberg RM. Arteriographic demonstration of external-internal carotid anastomosis through the ophthalmic arteries. Radiology. 1954;63(4):525-530. [DOI] [PubMed] [Google Scholar]
- 43. Wu WT. The Oriental nose: an anatomical basis for surgery. Ann Acad Med Singapore. 1992;21(2):176-189. [PubMed] [Google Scholar]
- 44. Zhang L, Pan L, Xu H, et al. Clinical observations and the anatomical basis of blindness after facial hyaluronic acid injection. Aesthetic Plast Surg. 2019;43(4):1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. LeSavage BL, Suhar NA, Madl CM, Heilshorn SC. Production of elastin-like protein hydrogels for encapsulation and immunostaining of cells in 3D. J Vis Exp. 2018;( 135):57739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jang JG, Hong KS, Choi EY. A case of nonthrombotic pulmonary embolism after facial injection of hyaluronic acid in an illegal cosmetic procedure. Tuberc Respir Dis (Seoul). 2014;77(2):90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tansatit T, Apinuntrum P, Phetudom T. An anatomical study of the middle temporal vein and the drainage vascular networks to assess the potential complications and the preventive maneuver during temporal augmentation using both anterograde and retrograde injections. Aesthetic Plast Surg. 2015;39(5):791-799. [DOI] [PubMed] [Google Scholar]
- 48. Kapoor KM, Bertossi D, Li CQ, Saputra DI, Heydenrych I, Yavuzer R. A systematic literature review of the middle temporal vein anatomy: “venous danger zone” in temporal fossa for filler injections. Aesthetic Plast Surg. 2020;44(5):1803-1810. [DOI] [PubMed] [Google Scholar]
- 49. Zhang LX, Lai LY, Zhou GW, et al. Evaluation of intraarterial thrombolysis in treatment of cosmetic facial filler-related ophthalmic artery occlusion. Plast Reconstr Surg. 2020;145(1):42e-50e. [DOI] [PubMed] [Google Scholar]
- 50. Zhang L, Feng X, Shi H, Wu WTL, Wu S. Blindness after facial filler injections: the role of extravascular hyaluronidase on intravascular hyaluronic acid embolism in the rabbit experimental model. Aesthet Surg J. 2020;40(3):319-326. [DOI] [PubMed] [Google Scholar]
- 51. Kumar N, Swift A, Rahman E. Development of “core syllabus” for facial anatomy teaching to aesthetic physicians: a Delphi consensus. Plast Reconstr Surg Glob Open. 2018;6(3):e1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17(6):1019-1024. [DOI] [PubMed] [Google Scholar]
- 53. Fabi SG, Goldman MP, Mills DC, et al. Combining microfocused ultrasound with botulinum toxin and temporary and semi-permanent dermal fillers: safety and current use. Dermatol Surg. 2016;42(Suppl 2):S168-S176. [DOI] [PubMed] [Google Scholar]
- 54. Schelke LW, Velthuis P, Kadouch J, Swift A. Early ultrasound for diagnosis and treatment of vascular adverse events with hyaluronic acid fillers. J Am Acad Dermatol. 2019;S0190-9622(19)32392-8. [DOI] [PubMed] [Google Scholar]