Table 1. Optimization Tablea.
| entry | variations from standard conditionsa | yield (%)b of 3aa |
|---|---|---|
| 1 | none | 97 (96)c |
| 2 | without PPh3 | NR |
| 3 | BINAP instead of PPh3 | NR |
| 4 | toluene instead of CHCl3 | 60 |
| 5 | DCM instead of CHCl3 | 78 |
| 6 | EtOAc instead of CHCl3 | 64 |
| 7 | CDCl3 instead of CHCl3 | 93 |
| 8 | 1 equiv of 1a | 46 |
| 9 | 2 equiv of 1a | 82 |
| 10 | 0.2 mmol 1a and 2 equiv of 2a | 20 |
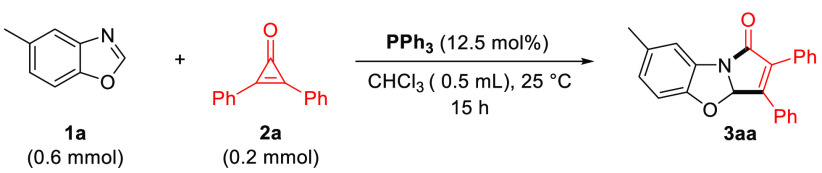
Unless otherwise noted, the standard reaction conditions were as follows: 1a (0.6 mmol), 2a (0.2 mmol), solvent (0.5 mL).
The yield was determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield.