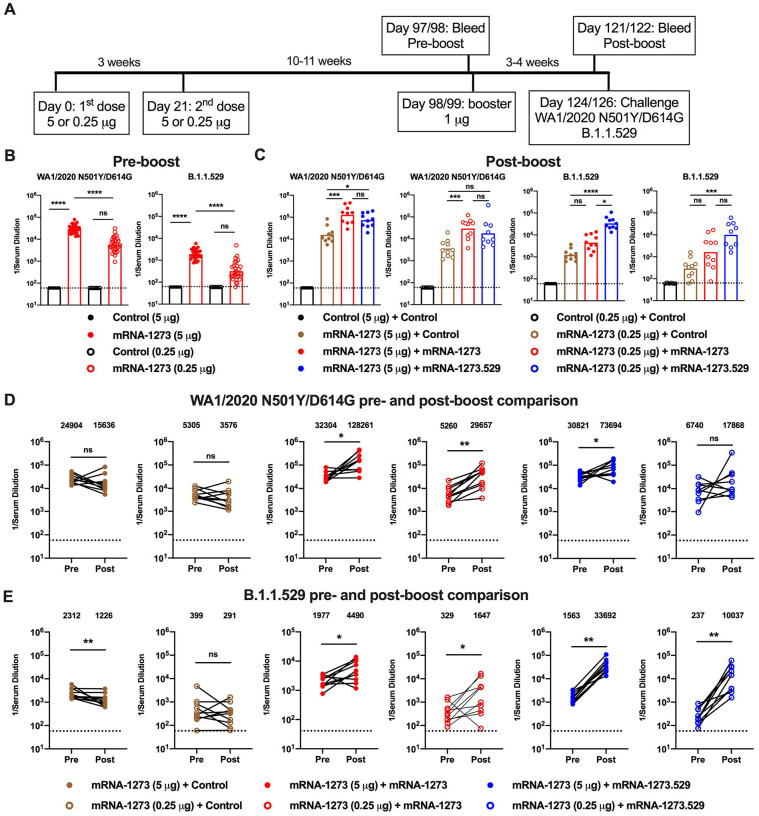
Figure 6. Booster doses of mRNA-1273 or mRNA-1273.529 enhance neutralizing antibody responses in 129S2 mice.
Seven-week-old female 129S2 mice were immunized with 5 or 0.25 μg of mRNA vaccines and then boosted 10 to 11 weeks later with 1 μg of control mRNA, mRNA-1273, or mRNA-1273.529. A. Scheme of immunizations, blood draws, and virus challenge. B-C. Serum neutralizing antibody responses immediately before (B, pre-boost) and three to four weeks after (C, post-boost) a control, mRNA-1273, or mRNA-1273.529 booster dose as judged by FRNT with WA1/2020 N501Y/D614G (left panel(s)) and B.1.1.529 (right panel(s)) in 129S2 mice that received primary series immunizations with 5 or 0.25 μg of control (n = 6) or mRNA-1273 (n = 30) vaccines (two experiments, boxes illustrate geometric mean values, dotted lines show the LOD). D-E. Paired analysis of pre- and post-boost serum neutralizing titers against WA1/2020 D614G (D) and B.1.1.529 (E) viruses from samples obtained from animals (data from B-C) that received the following primary and booster immunizations: mRNA-1273 (5 or 0.25 μg) + control booster, mRNA-1273 (5 or 0.25 μg) + mRNA-1273 booster, mRNA-1273 (5 or 0.25 μg) + mRNA-1273.529 booster (n = 10, two experiments, dotted lines show the LOD). GMT values are indicated at the top of the graphs. Statistical analyses. B-C: One-way ANOVA with Dunn’s post-test; D-E. Wilcoxon signed-rank test (ns, not significant; * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).