Abstract
Dyslipidemia-induced endothelial dysfunction is an important factor in the progression of cardiovascular disease; however, the underlying mechanisms are unclear. Our recent studies demonstrated that flow-induced vasodilation (FIV) is regulated by inwardly-rectifying K+ channels (Kir2.1) in resistance arteries. Furthermore, we showed that hypercholesterolemia inhibits Kir2.1-dependent vasodilation. In this study, we introduced two new mouse models: i) endothelial-specific deletion of Kir2.1 to demonstrate the role of endothelial Kir2.1 in FIV, and ii) cholesterol-insensitive Kir2.1 mutant to determine the Kir2.1 regulation in FIV under hypercholesterolemia. FIV was significantly reduced in endothelial-specific Kir2.1 knock-out mouse mesenteric arteries compared to control groups. In cholesterol-insensitive Kir2.1 mutant mice, Kir2.1 currents were not affected by cyclodextrin and FIV was restored in cells and arteries, respectively, with a hypercholesterolemic background. To extend our observations to humans, 16 healthy subjects were recruited with LDL-cholesterol ranging from 51–153 mg/dl and FIV was assessed in resistance arteries isolated from gluteal adipose. Resistance arteries from participants with >100 mg/dL LDL (high-LDL) exhibited reduced FIV as compared to those participants with <100 mg/dL LDL (low-LDL). A significant negative correlation was observed between LDL-cholesterol and FIV in high-LDL. Expressing dominant negative Kir2.1 in endothelium blunted FIV in arteries from low-LDL but had no further effect on FIV in arteries from high-LDL. The Kir2.1-dependent vasodilation more negatively correlated to LDL-cholesterol in high-LDL. Overexpressing WT Kir2.1 in endothelium fully recovered FIV in arteries from participants with high-LDL. Our data suggest that cholesterol-induced suppression of Kir2.1 is a major mechanism underlying endothelial dysfunction in hypercholesterolemia.
Keywords: endothelial Kir2.1, hypercholesterolemia, flow-induced vasodilation, resistance artery, cholesterol
Graphical Abstract

Introduction
Dyslipidemia-induced endothelial dysfunction plays a key role in the early stage of cardiovascular disease, the leading cause of death worldwide1–3. Most studies focused on the role of pro-inflammatory changes of endothelium in the development of atherosclerosis4. A number of earlier studies also established that dyslipidemia results in the impairment of endothelial-dependent control of blood flow in conduit brachial and coronary arteries5, 6. Specifically, it was shown in brachial arteries that the acetylcholine-induced increase in blood flow is significantly diminished in hypercholesterolemic patients as compared to healthy subjects5. Reduced blood flow rate was also observed in coronary arteries in hypercholesterolemic patients7. The key mediator of the vasodilatory response to flow is nitric oxide (NO)8 and since it is known that the bioavailability of NO is suppressed by hypercholesterolemia2; these observations suggest that hypercholesterolemic patients have increased vascular resistance attributed to a decreased availability of nitric oxide (NO)6. However, the mechanistic link between hypercholesterolemia and a decrease in NO production is not understood.
Our recent studies demonstrated that flow-induced production of NO and flow-induced vasodilation (FIV) critically depends on mechanically activated inwardly-rectifying K+ channels, Kir2.19. We also found that this pathway is distinct from one mediated by Ca2+-sensitive K+ channels that regulate FIV by an NO-independent mechanism. We also know from our previous studies that endothelial Kir2.1 channels are suppressed by loading the cells with cholesterol10, and by exposure to modified low-density lipoproteins (LDL)11. Significant suppression of endothelial Kir currents is also observed in endothelial cells freshly isolated from pigs fed high fat diet11 and from genetically hypercholesterolemic ApoE−/− mice12 leading to loss of Kir2.1 function12.
This study addresses a hypothesis that cholesterol sensitivity of endothelial Kir2.1 plays a major role in reduced FIV in resistance arteries of patients with elevated levels of LDL.
Methods
All supporting data are available within the article and supplementary results and detailed methods are in the Online Supplemental Materials. Also, please see the Major Resources Table in the Supplemental Materials for major resources.
Human subject recruitment
The study protocol and procedures were approved by the University of Illinois at Chicago Institutional Review Board (IRB#2016–0939). IRB approved the human experimental procedures based on the Declaration of Helsinki and good clinical practice.
All participants were between 21 and 55 years old. Exclusion criteria included: body mass index >33 kg/m2, a history of smoking, diabetes mellitus, cancer, heart, kidney or liver disease, gallbladder disease, autoimmune or other inflammatory disease. For females, who were not at the follicular phase were excluded. After written informed consent was obtained, biopsy samples of gluteal fat pads were collected at the University of Illinois Hospital and Health Sciences Center. Biopsies were placed in cold (4°C) HEPES buffer solution. Arteries were cleaned of fat and connective tissue and prepared for continuous measurement of internal luminal diameter as previously described9.
Statistics
Statistical analyses were performed using IBM SPSS Statistics (Version 27, Chicago, IL, USA). Data are presented as mean±StandardError or n.
To determine the correlations of maximal FIV or Kir2.1-dependent FIV component to outcomes of interests, Pearson product-moment correlation coefficients and scatter plots were used. To determine if LDL predicts maximal FIV or Kir2.1-dependent FIV component, multiple linear regression models were performed, adjusting for body mass index and age.
A paired or unpaired Student t test, 1-way ANOVA, or a 2-way with or without repeated measures ANOVA were used. Significance, in all cases, was set to P<0.05. Bonferroni post hoc tests were used to determine where differences existed after significance was detected with ANOVA.
Results
Endothelial-specific deletion of Kir2.1 results in significant decrease in FIV in murine mesenteric arteries
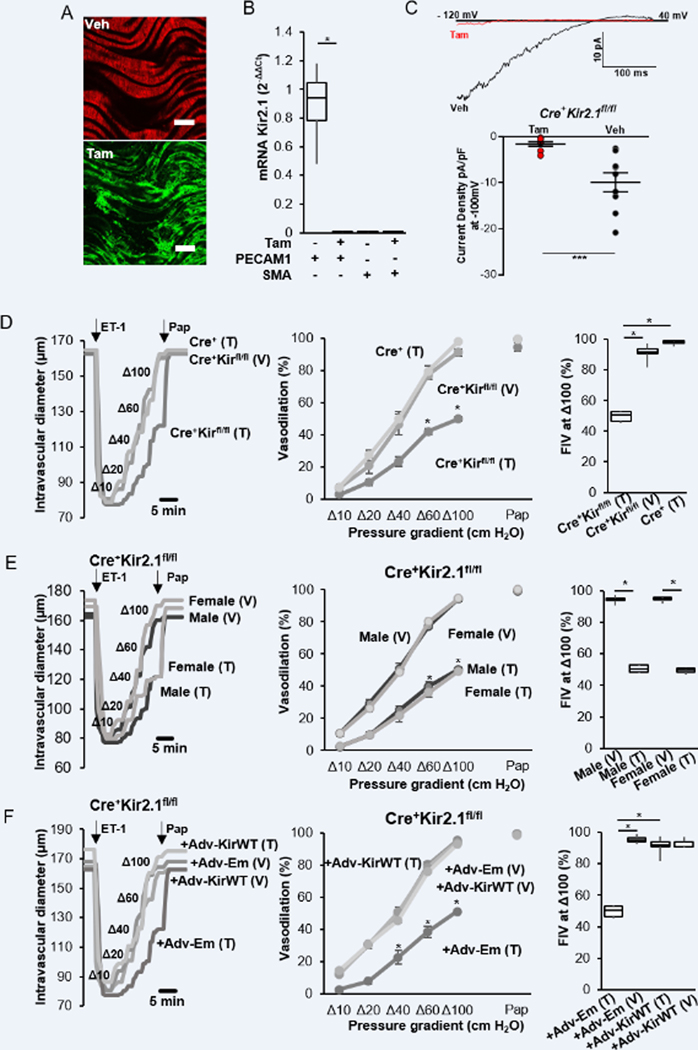
We have shown previously that global deficiency of Kir2.1 in Kir2.1+/− mice, as well as transfecting mouse arteries with dominant-negative Kir2.1 (dnKir2.1) construct driven by an endothelial-specific promoter (Cdh5), result in significant reduction in FIV9, 12. Our first objective here is to present direct evidence that endothelial-specific deletion of Kir2.1 is critical for FIV. To achieve this goal, a floxed Kir2.1 mouse model was generated on C57/BL6 background (scheme in figure S1A) and crossed with Cdh5.CreERT2 mouse, an inducible EC-specific Cre on the background of ROSAmT/mG mouse that allows detecting tamoxifen-induced activation of Cre by the convergence of tdTomato red fluorescent protein to GFP. This conversion is demonstrated in en face mouse mesenteric arteries (Fig. 1A). As shown previously9, 13, 14, Kir2.1 is significantly expressed in the endothelial but not in vascular smooth muscle cells in mouse mesenteric arteries (Fig. 1B). Deletion of Kir2.1 in endothelial cells was confirmed by the real-time PCR (Fig. 1B) and by electrophysiological recordings in freshly-isolated ECs showing a virtual loss of Kir2.1 functional expression (Fig. 1C).
Figure 1. Endothelial Kir2.1 regulates FIV.
A. Tamoxifen-induced eGFP expression in Cdh5.CreERT2ROSAmT/mG mouse mesenteric arteries, en face (scale bar=100 μm). B. Expression of Kir2.1 mRNA in freshly isolated mesenteric ECs from Cdh5.CreERT2Kir2.1fl/fl mice injected with tamoxifen or vehicle (n=4 pairs, *p<0.05). C. (Upper) Representative traces of Kir2.1 currents in freshly isolated mesenteric ECs from Cdh5.CreERT2Kir2.1fl/fl mice injected with tamoxifen or vehicle. (Bottom) Average of Kir 2.1 current densities with tamoxifen (n=9,10.1±2.7 pF) or vehicle (n=9, 16.1±1.4 pF, ***p<0.01). D. (Left) Representative traces of FIV in mesenteric arteries from Cdh5.CreERT2Kir2.1fl/fl with tamoxifen and vehicle injection, compared to Cdh5.CreERT2ROSAmT/mG injected with tamoxifen. (Middle) FIV in mesenteric arteries (n=8 pairs for tamoxifen and vehicle, n=6 for Cre control, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O from ii (*p<0.05). E. (Left) Representative traces of FIV in mesenteric arteries from male and female Cdh5.CreERT2Kir2.1fl/fl mice with tamoxifen or vehicle. (Middle) FIV of mesenteric arteries from male and female mice (n=4 groups). (Right) Comparison of FIV at Δ100 cmH2O from the middle (n=4 groups). F. (Left) Representative traces of FIV in mesenteric arteries from Cdh5.CreERT2Kir2.1fl/fl with tamoxifen or vehicle with Cdh5-WTKir2.1 or empty adenoviral constructs. (Middle) FIV of mesenteric arteries (n=8 pairs, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O from the middle (*p<0.05). (Pap: Papaverine, used to confirm no deterioration of the vascular smooth muscle dependent vasodilation.)
We show here that EC-specific deletion of Kir2.1 in Cdh5.CreERT2Kir2.1fl/fl mice injected with tamoxifen resulted in significant decrease in FIV, as compared to vehicle control (corn oil:ethanol, 10:1) or as compared to a Cre control (Cdh5.CreERT2ROSAmT/mG) injected with tamoxifen (Fig. 1D). Comparing the FIV in males vs. females of Cdh5.CreERT2Kir2.1fl/fl mice shows a full overlap of the FIV curves, both in mice injected with tamoxifen or vehicle, indicating that the contribution of Kir2.1 to FIV is similar in both sexes (Fig. 1E). Reduction of FIV in tamoxifen-injected Cdh5.CreERT2Kir2.1fl/fl mice was fully rescued with the over-expression of Kir2.1 WT construct driven by Cdh5 endothelial specific promoter, whereas exposing the arteries to a Cdh5 construct alone (Empty virus) had no effect (Fig. 1F). No effect of Kir2.1 overexpression on FIV was observed in arteries of control mice injected with the vehicle. These data directly show that endothelial Kir2.1 is critical for FIV in mouse resistance arteries. Endothelial-independent vasodilation was tested at the end of each FIV experiment by exposing the arteries to papaverine (10−4M), which is a well-known vascular smooth muscle relaxant9, 15, 16, a standard protocol in our laboratory9, 12, 17. None of the conditions described above had any effect on papaverine-induced vasodilation indicating that endothelial-independent response remains intact.
Hypercholesterolemia resistant FIV in CRISPR cholesterol insensitive Kir2.1-L222I mouse
Our previous structure-function studies of the cholesterol-sensitivity of Kir2.1 identified leucine at the position 222 as a critical residue whose substitution to isoleucine renders Kir2.1 insensitive to cholesterol18, 19. A CRISPR mouse was generated, therefore, by substituting leucine222 to isoleucine in C57/BL6 mouse (scheme in figure S2A). Furthermore, to determine whether endothelial Kir channels in CRISPR Kir-L222I mouse are cholesterol-insensitive, we crossed Kir2.1L222I mice with genetically hypercholesterolemic mice, ApoE−/−. Kir currents were recorded in ECs freshly-isolated from mesenteric arteries of WT, Kir2.1L222I, ApoE−/− and Kir2.1L222I/ApoE−/− mice. As we showed previously12, Kir currents in cells isolated from ApoE−/− mice are significantly smaller than in WT cells and here we demonstrate that the currents are fully rescued in Kir2.1L222I/ApoE−/− with no difference observed between WT and Kir2.1L222I (Fig. 2A). A lack of cholesterol sensitivity of Kir currents in endothelial cells from Kir2.1L222I mice is also confirmed by testing the effect of cholesterol depletion. As expected, Kir currents in WT ECs were significantly increased after the incubation with methyl-β-cyclodextrin (MβCD), known to remove cholesterol from the cell membrane12, but no MβCD -induced increase was observed in ECs isolated from Kir2.1L222I mesenteric arteries (figure S2B, C). These observations confirm that WT Kir2.1 was successfully replaced by its cholesterol insensitive mutant.
Figure 2. Cholesterol insensitive mutant of Kir2.1 recovers the cholesterol effect on impaired FIV in hypercholesterolemic mouse resistance arteries.
A. (Left) Representative traces of Kir2.1 currents in freshly isolated mesenteric ECs from WT, ApoE−/−, Kir2.1L222I, and ApoE−/−Kir2.1L222I mice. (Right) Average Kir current densities (WT:n=16 cells from 4 mice, 11.3±1.5pF, Kir2.1L222I:n=19 cells from 4 mice, 14.57±1.02pF, ApoE−/−:n=14 cells from 4 mice, 12.6±2.2pF, ApoE−/−Kir2.1L222I: n=14 cells from 4 mice, 12.5±1.2pF. *p<0.05 ***p < 0.005, ****p < 0.001). B. (Left) Representative traces of potassium currents of Kir2.1 recorded in freshly isolated mesenteric ECs from WT, ApoE−/−, Kir2.1L222I and ApoE−/−Kir2.1L222I mice with and without shear-stress. (Right) Average Kir current densities (WT:n=7 cells from 4 mice, 11.8±4.0pF, Kir2.1L222I:n=6 cells from 4 mice, 9.7±1.9pF, ApoE−/−:n=6 cells from 4 mice, 10.7±1.6pF, ApoE−/−Kir2.1L222I:n=6 cells from 4 mice, 12.3 ±2.2pF. *p<0.05, **p<0.01). C. (Left) Representative traces of FIV. (Middle) FIV of the mesenteric arteries from WT and Kir2.1L222I mice with empty (Em) and Cdh5-dnKir2.1 adenoviral vector (WT:n=10 mice, Kir2.1L222I:n=11, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O (*p<0.05). D. (Left) Representative traces of FIV. (Middle) FIV of the mesenteric arteries from ApoE−/− and Kir2.1L222I/ApoE−/− mice with empty (Em) and Cdh5-dnKir2.1 adenoviral vector (ApoE−/−:n=9, Kir2.1L222I/ApoE−/−:n=11, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O (*p<0.05).
Furthermore, Kir2.1L222I mutation also resulted in full rescue of the sensitivity of endothelial Kir channels to flow in ApoE−/− mice (Fig. 2B). Response of Kir currents to flow is measured in freshly-isolated cells seeded into a modified parallel plate chamber that allows electrophysiological recordings to be performed under well-defined flow conditions, as described in our earlier studies9, 12, 17, 20. As expected, Kir currents in WT cells show a typical increase in current density in response to flow, a response that virtually disappears in ApoE−/− cells. The flow response in Kir2.1L222I ECs is fully intact, similar to WT, and is fully restored in Kir2.1L222I/ApoE−/− cells.
Next, we tested whether abrogating cholesterol sensitivity of Kir2.1 also rescues FIV in hypercholesteremic ApoE−/− mice. As we have shown previously9, 12, in WT mesenteric arteries FIV is significantly decreased by transducing the arteries with Cdh5-dnKir2.1 construct, as compared to Empty virus control. We show here that FIV in mesenteric arteries isolated from Kir2.1L222I mice is fully functional and equivalent to FIV in WT arteries (Fig. 2C, compare FIV curves/dilation at Δ100 in WT and in Kir2.1L222I mice, both showing ~90 maximal dilation to flow). Furthermore, we also show that exposing Kir2.1L222I arteries to Cdh5-dnKir2.1 reduces FIV about ~2 folds, indicating that arteries from Kir2.1L222I mice have strong Kir2.1-dependent FIV component, similar to that in WT arteries (Fig. 2C, compare FIV curves/maximal dilations in WT and Kir2.1L222I mice exposed to Cdh5-dnKir2.1 (DN)).
In mesenteric arteries isolated from ApoE−/− mice, FIV is reduced about 2-fold with no further decrease in response to Cdh5-dnKir2.1 (Fig. 2D), as was also shown in our previous study12. Most importantly, we found that the vasodilation of arteries isolated from Kir2.1L222I/ApoE−/− mice is fully responsive to shear stress, in spite of the hypercholesterolemic background, indicating that the substitution of Kir2.1 with its cholesterol insensitive mutant results in full rescue of FIV in hypercholesterolemia (Fig. 2D: compare FIV in ApoE−/− mice that show ~40% maximum dilation to flow with FIV in Kir2.1L222I/ApoE−/− mice that show more than 90% maximum dilation). Finally, unlike ApoE−/− mice, arteries isolated from Kir2.1L222I/ApoE−/− mice are also sensitive to Cdh5-dnKir2.1, further verifying that Kir2.1-dependent vasodilation is fully restored in Kir2.1L222I/ApoE−/− mice (Fig. 2D: compare FIV in Kir2.1L222I/ApoE−/− exposed to Cdh5-dnKir2.1 (DN) that show significant decrease in the dilation response, as compared to Kir2.1L222I/ApoE−/− exposed to the empty virus, with FIV in ApoE−/− exposed to Cdh5-dnKir2.1 that show no further decrease relative to ApoE−/− exposed to the empty virus). No differences are observed in papaverine-induced vasodilation for any of the mouse groups or treatments.
Our next goal was to determine whether FIV of resistance arteries is impaired in human subjects with elevated levels of LDL and whether endothelial Kir2.1 plays a significant role in this process.
Decrease in FIV in human resistance arteries as a function of LDL
As described previously9, FIV was measured by generating a pressure gradient (Δ10-Δ100 cmH2O) in isolated adipose resistance arteries. After a 12-hour fast with no use of caffeine, alcohol, and medication, a gluteal fat biopsy was obtained from a group of 20 healthy subjects with plasma LDL levels ranging from 51 to 153 mg/dl and total cholesterol ranging from 112 to 231 mg/dl (table S1). The lipid panel was determined in venous blood samples collected on the day of the biopsy. Resistance arteries were then isolated from the fat tissues and FIV was performed by research personnel without prior knowledge of the results of the lipid panel. As described above for the murine vessels, at the end of each FIV measurement, papaverine (10−4M) was applied to induce endothelial-independent vasodilation.
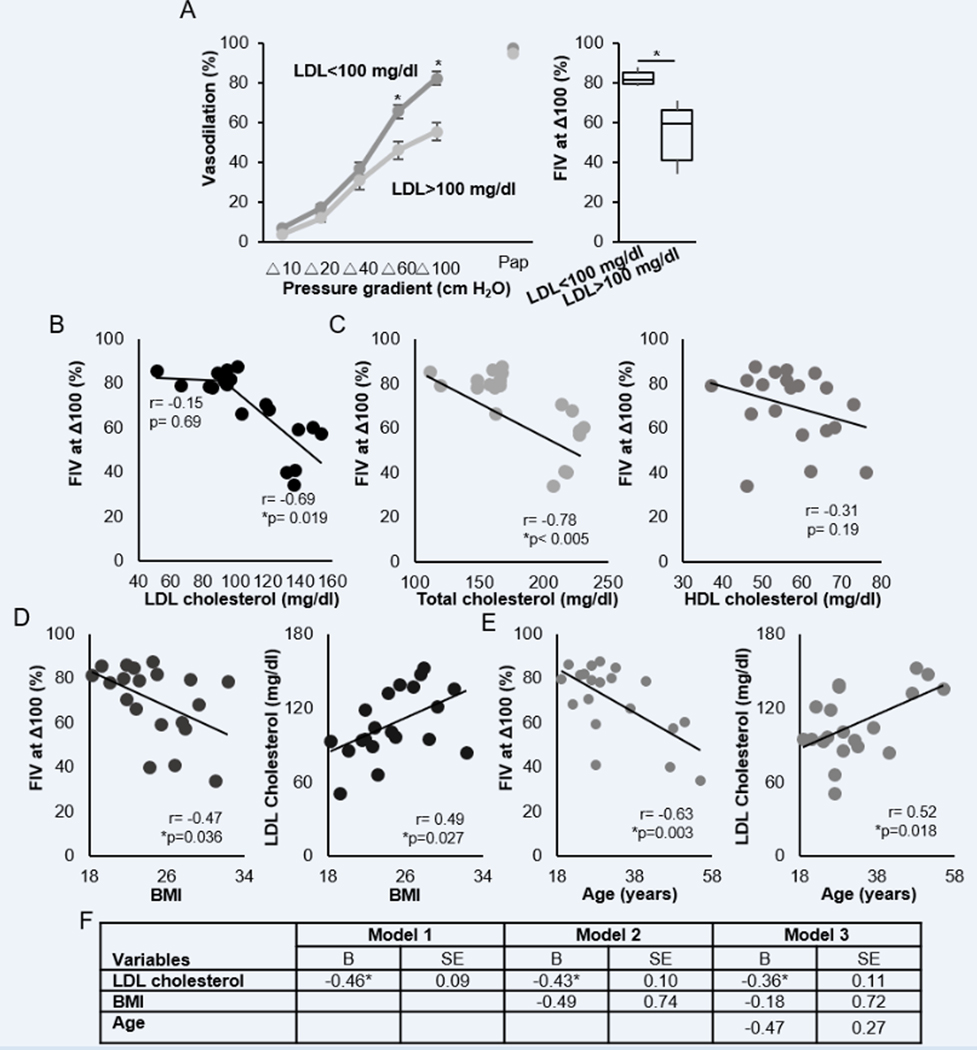
To determine whether the degree of the vasodilation correlates with the levels of plasma LDL, we separated the subjects into a low LDL group (plasma LDL<100 mg/dl, 11 subjects) and a high LDL group (>100 mg/dl, 9 subjects), the target range for LDL cholesterol according to recent AHA guidelines21–23. The two groups differed significantly in the levels of total and LDL cholesterol, LDL/HDL ratios and triglycerides but not in the level of HDL, glucose, insulin or body mass index (BMI) (Table 1). A significant reduction in the FIV in the high LDL group was observed at Δ60 cmH2O and Δ100 cmH2O pressure gradients (Fig. 3A). There was no reduction in response to papaverine, an endothelial-independent vasodilator, indicating that a reduction in FIV is due to an impairment of the endothelial response. Maximal flow-induced dilations for individual subjects showed a negative correlation with the LDL level for subjects with LDL ranging between 90 and 160 mg/dl based on Pearson correlation (Fig. 3B; no correlation was observed below 100 mg/dl LDL). A negative correlation was also observed between FIV and total cholesterol but no correlation was found between FIV and HDL (Fig. 3C) indicating that LDL, but not HDL, was a major factor for FIV in these subjects. Also, no correlation was observed between FIV and triglycerides, glucose or insulin (figure S3A–C), as well as between males and females (figure S4A), and which were also similar in the levels of LDL (figure S4B, C) and all other parameters. However, FIV was found to be negatively correlated with BMI (Fig. 3D) and with age (Fig. 3F; both BMI and age positively correlated with LDL (Fig. 3E,G). Using multiple linear regression models with FIV as a dependent variable and LDL, BMI, and age as independent variables (Fig. 3F), LDL was a predictor of FIV (model 1, p<0.0005), even when adjusted for BMI (model 2, p=0.001), as well as for both BMI and age (model 3, p=0.004).
Table 1. Subject characteristics.
Data are mean±SE or n. BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
| Measure | mean±SE | mean±SE | p-value |
|---|---|---|---|
| LDL level, mg/dl | <100 | >100 | |
| Sex, M/F | 8/3 | 4/5 | |
| AGE, years | 29.6 ± 2.5 | 38.0 ± 4.1 | 0.106 |
| Height, Inches | 71.3 ± 0.8 | 66.8 ± 1.3 | 0.015 |
| Weight, lbs | 161.6 ± 10.2 | 164.4 ± 5.7 | 0.820 |
| BMI, kg/m2 | 23.6 ± 1.6 | 26.3 ± 1.0 | 0.186 |
| LDL Cholesterol, mg/dl | 82.3 ± 6.6 | 132.1 ± 5.1 | *0.0001 |
| HDL Cholesterol, mg/dl | 53.1 ± 3.1 | 61.2 ± 3.6 | 0.110 |
| Total Cholesterol, mg/dl | 89.4 ± 9.0 | 152.9 ± 5.7 | *0.003 |
| Glucose, mg/dl | 136.5 ± 12.5 | 214.1 ± 6.9 | 0.578 |
| Insulin, μIU/ml | 91.2 ± 2.2 | 93.6 ± 3.5 | 0.772 |
| Triglycerides, mg/dl | 6.0 ± 0.5 | 5.5 ± 1.4 | *0.034 |
| Systolic BP, mmHg | 118.8 ± 3.2 | 118.3 ± 2.1 | 0.89 |
| Diastolic BP, mmHg | 73.1 ± 1.4 | 76.1 ± 1.9 | 0.23 |
| Vessel diameter, μm | 108.8 ± 11.2 | 125.4 ± 9.4 | 0.26 |
Figure 3. The effect of elevated LDL cholesterol in FIV of human resistance arteries.
A. (Left) FIV in human subjects with lower than 100 mg/dl LDL cholesterol (n=11) and higher than 100 mg/dl LDL cholesterol (n=9, *p<0.05) (Right) FIV at Δ100 cmH2O. (*p<0.05) B. Correlation of LDL cholesterol and FIV at the pressure gradient of Δ100 cmH2O (n=16, *p<0.05). C. (Left) Correlation of total cholesterol and FIV at the pressure gradient of Δ100 cmH2O (n=16). (Right) Correlation of HDL cholesterol and FIV at the pressure gradient of Δ100 cmH2O (n=16). D. (Left) Correlation of BMI and FIV at the pressure gradient of Δ100 cmH2O (n=16, *p<0.05) (Right) Correlation of LDL cholesterol and BMI (n=16, *p<0.05). E. (Left) Correlation of age and FIV at the pressure gradient of Δ100 cmH2O (n=16, *p<0.05). (Right) Correlation of LDL cholesterol and age (n=16). (Correlation Coefficient (r)) F. Multiple linear regression models using enter method of factors of interest (LDL, BMI, and age) to predict FIV at the pressure gradient of Δ100 cmH2O in healthy adults (n=20). B=Unstandardized coefficients; BMI=body mass index; LDL=low-density lipoprotein; SE=standard error (*p <0.05).
Loss of Kir2.1 contribution to FIV in human resistance arteries as a function of LDL
Suppression of endothelial Kir by LDL:
Our previous studies showed that endothelial Kir currents are significantly suppressed in porcine and murine hypercholesterolemic models11, 12. The effect of LDL exposure, however, has not been determined. Here, we test the impact of LDL on Kir currents in human aortic endothelial cells (HAECs) and show that exposing HAECs to the elevated levels of LDL (150–250 mg/dl) resulted in a significant increase in the level of cellular cholesterol and suppression of Kir currents. More specifically, exposing HAECs to 75 mg/dl LDL had no effect on cellular cholesterol nor on Kir currents, but increasing LDL to 150–250 mg/dl resulted in an increase in cellular free cholesterol (Fig. 4A(left)) and decrease in Kir current density (Fig. 4A(right)). An increase in cellular cholesterol in endothelial cells exposed to high levels of LDL is consistent with our recent study24. The negative impact of cellular cholesterol on endothelial Kir currents is well-established in our laboratory11, 12.
Figure 4. The role of endothelial Kir2.1 in human resistance arterial FIV with elevated LDL cholesterol.
A. (Left) Cholesterol loading in HAECs after the incubation with 0, 75, 150, and 250 mg/dl LDL for 48 hours. (n=3, *p<0.05) (Right) Current density of Kir2.1 at −100 mV after the incubation with 0, 75, 150, and 250 mg/dl LDL for 48 hours. (n=10–14, *p<0.05) B. (Left) FIV in human subjects with lower than 100 mg/dl LDL cholesterol after 48 hrs incubation with empty viral vector (Em) and Cdh5-dnKir2.1 adenoviral vector (DN) with and without Ba2+ (Ba) (n=11, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O (n=11, *p<0.05). C. (Left) FIV in human subjects with higher than 100 mg/dl LDL cholesterol under same condition with A (n=9). (Right) Comparison of FIV at Δ100 cmH2O (n=9). D. Correlation of LDL versus Kir-dependent (Left) and Kir-independent (Right) FIV at Δ100 cmH2O (n=20). E. Correlation of FIV and Kir-dependent FIV at Δ100 cmH2O (n=16). (Kir-dependent FIV is defined as Ba2+-(Δ) and Cdh5-dnKir2.1(o)-sensitive FIV. Kir-independent FIV is defined as Ba2+-(Δ) and Cdh5-dnKir2.1(o)-resistant FIV. Correlation Coefficient (r) and *p<0.05)) F. Multiple linear regression models using enter method of factors of interest (LDL, BMI, and age) to predict Kir2.1-dependent maximal FIV in healthy adults (n=20). B=Unstandardized coefficients; BMI=body mass index; LDL=low-density lipoprotein; SE=standard error (*p <0.05).
Contribution of Kir2.1 to FIV in subjects with low and high LDL:
Our next question was whether the impairment of FIV in human subjects with hypercholesterolemia can be attributed to the loss of function of endothelial Kir2.1 channels. To address this question, isolated adipose resistance arteries from the same group of subjects (Table 1) were exposed to Cdh5-dnKir2.1 and control arteries from the same biopsies were exposed to an empty adenovirus construct (Empty virus). In the low-LDL group, the FIV was significantly decreased by about 2-fold in arteries exposed to Cdh5-dnKir2.1 as compared to the controls (Fig. 4B), as expected based on our previous studies12. No deterioration of the vasodilatory response occurred after the arteries were incubated with the empty virus for 48h, as is indicated by testing the reactivity of the vessels both flow and Acetylcholine (figure S5A, B). Exposure of the arteries to Ba2+ or ML133, the two known blockers of Kir channels9, 25 also resulted in a significant decrease in FIV in control arteries with no difference observed between the effects of Ba2+, ML133 or a combination of the two (figure S5C). No difference was also observed between the arteries exposed to Ba2+ or transduced with Cdh5-dnKir2.1, confirming that the functional expression of the channels is key to the FIV response in the low-LDL group of patients. In contrast, in the high-LDL group, in which FIV was impaired, exposing the arteries to Cdh5-dnKir2.1 had no effect with dnKir2.1-transduced arteries and arteries exposed to an empty virus showing identical degree of FIV at all pressure levels (Fig. 4C). Application of Ba2+ had no effect on any of the FIV in arteries in the high-LDL group. Application of papaverine induced full dilation in all vessels in this study, as shown in our previous studies9, 12, 17. Maximal dilations (FIV at Δ100 cmH2O) for low-LDL and high-LDL groups are shown in Fig. 4B(right), C(right). These data indicate that the functional contribution of Kir2.1 to FIV is lost in the endothelium of arteries isolated from the biopsies in high-LDL group further suggesting that impairment of Kir channels results in the impairment of FIV.
The Kir2.1-dependent component of FIV was calculated by subtracting maximal FIV in vessels either exposed to Cdh5-dnKir2.1 (circles) or to Ba2+ (triangles) from maximal FIV in control vessels from the same biopsy. In the subjects with the LDL levels below 100 mg/dl, the Kir2.1-dependent component of FIV was about 40% of the dilatory contribution in arteries from subjects with higher LDL levels. At >120 mg/dl LDL levels, there was a sharp reduction in the Kir2.1-dependent component of FIV. (Fig. 4D(left)). Notably, the Kir2.1-independent component of the FIV, defined as the residual FIV portion after Kir2.1 inhibition, did not show a negative correlation with the LDL level (Fig. 4D(right)) suggesting that the impairment of the FIV in subjects with high LDL should be specifically attributed to the Kir2.1-dependent FIV. Instead, we observed that a Kir2.1-independent component slightly increased at high LDL levels suggesting that it might be a partial compensatory effect. Finally, plotting the Kir2.1-dependent FIV component of the vessel as a function of the maximal FIV of the same vessel show that healthy vessels that are capable of fully dilating have strong Kir2.1 component whereas vessels with a reduced Kir2.1 component have a low FIV or none at all (Fig. 4E). This result supports the mechanistic connection between the loss of Kir2.1 and the impairment of FIV in hypercholesterolemic patients. To determine if LDL was a predictor of Kir2.1-independent component of FIV, multiple linear regression models were performed with Kir2.1-independent component of FIV as dependent variable and LDL as independent variable (Fig. 4F). We found that LDL cholesterol was a predictor of Kir2.1-dependent FIV (model 1, P<0.0005), even when adjusted for BMI (model 2, p=0.002), and adjusted for both BMI and age (model 3, p=0.007). Therefore, these results imply that Kir2.1 is the key component affected by LDL, which fails to contribute to FIV in the presence of elevated LDL cholesterol. The effects hold for both LDL and total cholesterol. Specifically, we show that FIV/Kir2.1-dependent FIV component correlate negatively with both LDL level and total cholesterol level in the plasma. This is indeed expected because the level of total cholesterol and the levels of LDL strongly correlate indicating that it is the increase in LDL that underlies an increase in total cholesterol in these subjects. This is also consistent with a lack of correlation between FIV/Kir2.1-dependent FIV component and HDL, another major component of plasma cholesterol. Taken together, these data suggest that it is the increase in LDL that is the cause of the impairment of the FIV via the loss of Kir2.1 contribution to the flow response (Fig. 3C, figure S6A–C, table S2,S3). No correlation was observed between either of the FIV components and triglycerides, glucose or insulin (figure S6D–F).
Rescue of FIV at high LDL with EC-specific WT-Kir2.1:
To determine whether impaired FIV in hypercholesterolemic patients can be rescued by increased expression of Kir2.1 channels in endothelial cells, a set of arteries from both low-LDL and high-LDL groups were exposed to adenoviral constructs expressing wild type Kir2.1 (WTKir2.1) driven by the endothelial-specific Cdh5 promoter. These data show that over-expression of Kir2.1 in endothelial cells of arteries isolated from the biopsies of hypercholesterolemic patients resulted in a full rescue of the FIV (Fig. 5A) indicating that the loss of Kir2.1 functional expression is the key factor in the impairment of FIV in these patients. In contrast, over-expression of Kir2.1 had no effect on FIV of the arteries isolated from the biopsies of subjects from the low-LDL group (Fig. 5B) indicating that Kir2.1 activity is not a limiting factor of the FIV in this group.
Figure 5. Recovery of FIV under elevated LDL cholesterol by overexpression of endothelial WT Kir2.1 in human resistance arteries.
A. (Left) FIV of the resistance arteries from human subjects with below 100 mg/dl LDL with empty (Em) and Cdh5-WTKir2.1 (WT) adenoviral vectors with and without Ba2+ (Ba) (n=7, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O (n=7, *p<0.05). B. (Left) FIV of the resistance arteries from human subjects with above 100 mg/dl LDL with empty (Em) and WT Kir2.1 overexpression (WT) adenoviral vectors with and without Ba2+ (Ba) (n=9, *p<0.05). (Right) Comparison of FIV at Δ100 cmH2O (n=9, *p<0.05).
Discussion
There are several novel findings reported in this study. First, we demonstrate that inducible endothelial-specific deletion of Kir2.1 results in significant reduction of FIV in mouse mesenteric arteries, providing strong validation to our previous studies showing the FIV impairment with the downregulation of Kir2.1 with a dominant-negative viral construct. Furthermore, using CRISPR gene editing, we found that a single-point mutation in Kir2.1 substituting leucine 222 for isoleucine rescues endothelial Kir currents and their mechanosensitivity to flow under hypercholesterolemic conditions. Furthermore, CRISPR gene editing that rendered a Kir2.1 insensitive to cholesterol protects FIV in mutant mice from hypercholesterolemia-induced dysfunction. This study also presents the first direct evidence that elevated levels of plasma LDL leads to severe suppression of flow-induced vasodilation in resistance arteries of humans. We found that the LDL-induced impairment of FIV is the result of the functional loss of endothelial inwardly rectifying K+ channels and show that a full rescue of FIV under hypercholesterolemic conditions can be achieved by over-expressing Kir2.1 in human arteries using viral transfection techniques in isolated arterioles.
Our previous studies demonstrated that cholesterol directly binds to Kir channels26 via the interaction between cholesterol molecules and the hydrophobic pockets of the channel protein and identified specific residues responsible for the sensitivity of Kir2.1 to cholesterol27, 28. Based on these structural studies, we identified a substitution of leucine at position 222 with isoleucine to render the channels completely cholesterol insensitive while fully preserving the normal activity of the channels18, 29. Further analysis established that this residue is not part of cholesterol binding sites of Kir2.1 but rather a part of an intramolecular mechanism that couples cholesterol binding with channel gating19, 28. A complete loss of cholesterol sensitivity of the channels together with normal functional activity made it an ideal candidate for the gene editing to determine the role of cholesterol sensitivity of these channels in vascular function. Therefore, we generated Kir2.1L222I mutant mice and confirmed that, as expected, Kir currents in freshly-isolated mesenteric endothelial cells of Kir2.1L222I mice are not cholesterol sensitive. Kir current densities in endothelial cells isolated from Kir2.1L222I mice were similar to those in WT mice, showing that even though they lost their cholesterol sensitivity, the current density did not increase. The most likely explanation is that in addition to the abrogation of cholesterol sensitivity, this mutation may have other subtle effects on channel activity, as was shown for multiple other mutations19, 28. To test the impact of Kir2.1L222I mutation on cholesterol-induced impairment of FIV, Kir2.1L222I mice were crossed with ApoE−/−, which we showed previously to have diminished FIV12. Our new data show that rendering Kir2.1 channels cholesterol insensitive preserved Kir2.1 activity and response to flow in ApoE−/− mice, and fully prevented the damage to FIV in this hypercholesterolemic model. These observations provide unequivocal evidence that cholesterol-sensitivity of Kir2.1 is the major factor in impaired FIV during hypercholesterolemia.
In humans, earlier studies found that hypercholesterolemia has a detrimental effect on blood flow in brachial arteries5, 6, 30, 31. It was shown that hypercholesterolemic patients had significantly reduced maximum blood flow response to acetylcholine5, 6 and following forearm ischemia30, 31. These studies were performed with non-invasive techniques used for large arteries (above 1mm diameter). It is known, however, that it is the resistance arteries with diameters of 50–200 μm that play the dominant role in the control of blood pressure and vascular tone32, 33. Therefore, we used an alternative approach of isolating resistance arteries from biopsies of gluteal fat of human subjects with and without hypercholesterolemia in order to test flow-induced vasodilation ex vivo. This approach has been widely used to assess endothelium-dependent vasodilation in obesity, hypertension, and diabetes16, 34, 35, but not in hypercholesterolemia, except for one earlier study that found no effect36. In contrast, our data show a strong negative correlation between plasma LDL and FIV that was independent from BMI and from age, indicating that the plasma level of LDL is a significant contributor to impairing FIV in resistance arteries. It is important to note that lower shear stress used in the previous study (below 10 dyn/cm2) that found no effect of hypercholesterolemia compared to our study which used shear of ~20 dyn/cm2 may help to explain differences between the two studies. Indeed, we also see no difference between low-LDL and high-LDL groups at low shear stress levels induced with pressure gradients less than 60 cmH2O. It is also important to consider the role of LDL in cholesterol loading of endothelial cells. Earlier studies in macrophages found that exposure to high levels of LDL does not lead to significant cholesterol loading, which was attributed to the downregulation of the LDL receptors37and it was proposed that cholesterol loading is mediated by modified forms of LDL that are recognized by different types of scavenger receptors38. Our work in endothelial cells, however, demonstrated that exposure to high level of LDL does lead to significant cholesterol loading and cholesterol-induced changes in membrane structure24. Similarly, we show here that exposure to the high levels of LDL resulted in both, a significant increase in the level of cellular cholesterol and suppression of Kir current density in human aortic endothelial cells. However, it is not possible currently to discriminate between the effects of LDL and its modified forms on the physiological effects observed in vivo or ex vivo, particularly in human subjects and new methodological approaches should be developed to achieve this goal.
Our study provides significant new insights into the mechanism of hypercholesterolemia-induced impairment of FIV. Based on our earlier studies in mice12, we hypothesized that cholesterol-induced suppression of endothelial Kir2.1 underlies the impairment of FIV in human patients with elevated LDL. Here, we present multiple lines of evidence that this is indeed the case: (i) exposure to physiological levels of elevated LDL, which we recently showed to increase cellular cholesterol in HAECs24, results in significant decrease in Kir2.1 currents; (ii) functional downregulation of endothelial Kir2.1 impairs FIV in arteries of subjects with low/normal LDL (below 100 mg/dl) and has no significant effect on FIV in subjects with LDL above 100 mg/dl; (iii) the Kir2.1-dependent FIV component decreases progressively with the level of LDL independently of BMI and age; (iv) an impaired FIV in hypercholesterolemic subjects is fully rescued by the over-expression of Kir2.1. Taken together, these observations indicate that suppression of endothelial Kir2.1 plays a central role in driving the impairment of FIV in patients with hypercholesterolemia. Notably, we demonstrate here that cholesterol-induced inhibition is specific for Kir2.1-dependent FIV whereas Kir2.1-independent component of FIV is not cholesterol sensitive.
Limitations of the study
A limitation of this study is that we use ET-1 rather than pressure-induced preconstruction, which is a more physiological approach. However, we used ET-1 to pre-constrict the mouse mesenteric arteries since 1) the mesenteric pressure induced constriction is less consistent than other vascular beds and 2) to maintain a close translational element to our human studies where intraluminal pressure will not induce the desired levels of constriction for subsequent assessment of dilatory function. This is a method that we routinely use in our studies9, 12, 17.
Another limitation is that the mechanistic strengths of the human studies are limited to associations, a frequent limitation in pilot studies with human subjects. This is mostly due to limited tissue availability for single blood vessel preparations in humans. On the other hand, combining human vessels in patients with high cholesterol is just the type of translational analysis needed to move to a full clinical intervention involving Kir targets. Given the strong associations observed between cholesterol and Kir in contributing to microvascular FIV in this study, our future directions will include further mechanistic analysis and exploring possible therapeutic interventions.
Perspectives:
Hypercholesterolemia is a risk factor for hypertension but the mechanistic link between hypercholesterolemia and hypertension is unclear. Here, we present novel evidence that cholesterol sensitivity of Kir2.1 is a major contributor to reduced FIV in hypercholesterolemic mice and humans. Using two novel mouse models, endothelial-specific deletion of Kir2.1 and a gene editing CRISPR substituting Kir2.1 with its cholesterol-insensitive mutant, we demonstrate that: (i) endothelial specific deletion of Kir2.1 significantly reduces FIV in a reversible way and (ii) rendering the channels to be cholesterol-insensitive prevents hypercholesterolemia-induced reduction of FIV. In humans, FIV of resistance arteries negatively correlates with LDL, with the reduction in FIV in hypercholesterolemic subjects attributed to the loss of Kir2.1 function, whereas endothelial-specific overexpression of Kir2.1 results in full rescue of FIV in these subjects.
Clinical relevance:
These findings build a potential foundation for a novel therapeutic strategy to alleviate the negative impact of hypercholesterolemia on blood pressure. Reduction in FIV is a hallmark of endothelial dysfunction and increase in peripheral resistance, which is a critical factor in the development of hypertension. Notably, we found that the critical LDL level that appears to trigger the detrimental process in Kir2.1-dependent component of FIV is 100 mg/dl, a level that is currently recommended as a target level for patients by American Heart Association14–16. We propose that further studies to identify inhibitors and other novel interventions that interfere with cholesterol binding to Kir2.1 channels may be a critical area of investigation to improve microvascular function, specifically for hypercholesterolemic patients.
Supplementary Material
Novelty and Significance.
What is new?
Two new novel mouse models are introduced in this study: 1) EC-specific Kir2.1 KO and 2) cholesterol-insensitive Kir2.1L222I mouse models.
What is relevant?
ex vivo FIV measurement represents physiological vasodilatory potential in vivo, which affects peripheral resistance, a major factor in hypertension. This study shows that cholesterol-induced FIV reduction is due to the cholesterol-sensitivity of Kir2.1, which will result in increasing peripheral resistance.
Summary
This study suggests that Kir2.1 is a possible candidate, which connects hypercholesterolemia and hypertension.
Acknowledgements
We are grateful to Dr. Yulia Epshtein for helping with collecting data and performance of the experiments with human aortic endothelial cells, Dr. Nicholas Barbera and Gregory Kowalsky for helping the graphical designs, Ms. Melissa Rader and Aleema Jackson for recruiting and collecting clinical information from the participants, CRC core for collecting biopsies and blood samples, Ms. Maureen Policastro for assisting by designing and producing our transgenic mouse model, and Ms. Crystal Adamos for helping with some of the experiments.
Sources of Funding
This research is supported by R01HL073965 (IL), R01HL141120 (IL), R01HL083298 (IL), T32HL144909 (SJA, ISF), and T32HL007829 (ISF) from NHLBI, T32DK080674 (ISF) from NIDDK, General University Research Program from University of Delaware (ISF), P20GM113125-6564 (ISF) and DE INBRE Award supported by P20GM103446 (ISF) from NIGMS.
Nonstandard Abbreviation and Acronyms
- Cdh5
Vascular-endothelial cadherin gene
- DBP
Diastolic blood pressure
- EC
Endothelial cells
- FIV
Flow-induced vasodilation
- HAEC
Human aortic endothelial cells
- Kir2.1
Inwardly-rectifying potassium channel family 2 subtype 1
- MβCD
methyl-β-cyclodextrin
- SBP
Systolic blood pressure
Footnotes
Disclosures
All authors report no conflicts.
Reference
- 1.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wever R, Stroes E, Rabelink TJ. Nitric oxide and hypercholesterolemia: A matter of oxidation and reduction? Atherosclerosis. 1998;137 Suppl:S51–60 [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson IB, Cockcroft JR. Cholesterol, endothelial function and cardiovascular disease. Curr Opin Lipidol. 1998;9:237–242 [DOI] [PubMed] [Google Scholar]
- 4.Le Master E, Levitan I. Endothelial stiffening in dyslipidemia. Aging (Albany NY). 2019;11:299–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilligan DM, Guetta V, Panza JA, Garcia CE, Quyyumi AA, Cannon RO 3rd. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994;90:35–41 [DOI] [PubMed] [Google Scholar]
- 6.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547 [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann PA, Gnecchi-Ruscone T, Schafers KP, Luscher TF, Camici PG. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36:103–109 [DOI] [PubMed] [Google Scholar]
- 8.Vanhoutte PM, Boulanger CM, Mombouli JV. Endothelium-derived relaxing factors and converting enzyme inhibition. Am J Cardiol. 1995;76:3E–12E [PubMed] [Google Scholar]
- 9.Ahn SJ, Fancher IS, Bian JT, Zhang CX, Schwab S, Gaffin R, Phillips SA, Levitan I. Inwardly rectifying k+ channels are major contributors to flow-induced vasodilatation in resistance arteries. J Physiol. 2017;595:2339–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward rectifier k+ current by optical isomers of cholesterol. Biophys. J. 2002;83:3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Mohler ER III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying k+ channels in aortic endothelium in vitro and in vivo. Circ Res. 2006;98:1064–1071 [DOI] [PubMed] [Google Scholar]
- 12.Fancher IS, Ahn SJ, Adamos C, Osborn C, Oh MJ, Fang Y, Reardon CA, Getz GS, Phillips SA, Levitan I. Hypercholesterolemia-induced loss of flow-induced vasodilation and lesion formation in apolipoprotein e-deficient mice critically depend on inwardly rectifying k(+) channels. J Am Heart Assoc. 2018;7:pii: e007430. doi: https://007410.001161/JAHA.007117.007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier k channels in the rat isolated mesenteric artery. J Vasc Res. 2003;40:159–168 [DOI] [PubMed] [Google Scholar]
- 14.Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. Kir channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai S, Kitagawa T. A comparison of the differential effects of nitroglycerin, nifedipine and papaverine on contractures induced in vascular and intestinal smooth muscle by potassium and lanthanum. Jpn J Pharmacol. 1981;31:193–199 [DOI] [PubMed] [Google Scholar]
- 16.Robinson AT, Fancher IS, Sudhahar V, Bian JT, Cook MD, Mahmoud AM, Ali MM, Ushio-Fukai M, Brown MD, Fukai T, Phillips SA. Short-term regular aerobic exercise reduces oxidative stress produced by acute in the adipose microvasculature. Am J Physiol Heart Circ Physiol. 2017;312:H896–H906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fancher IS, Le Master E, Ahn SJ, Adamos C, Lee JC, Berdyshev E, Dull RO, Phillips SA, Levitan I. Impairment of flow-sensitive inwardly rectifying k(+) channels via disruption of glycocalyx mediates obesity-induced endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2020;40:e240–e255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epshtein Y, Chopra A, Rosenhouse-Dantsker A, Kowalsky G, D.E. L, Levitan I. Identification of a c-terminus domain critical for the sensitivity of kir2.1 channels to cholesterol.. PNAS. 2009;106:8055–8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenhouse-Dantsker A, Logothetis DE, Levitan I. Cholesterol sensitivity of kir2.1 is controlled by a belt of residues around the cytosolic pore. Biophysical Journal. 2011;100:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitan I, Helmke BP, Davies PF. A chamber to permit invasive manipulation of adherent cells in laminar flow with minimal disturbance of the flow field. Ann Biomed Eng. 2000;28:1184–1193 [DOI] [PubMed] [Google Scholar]
- 21.Robinson JG. Lipid management beyond the guidelines. Prog Cardiovasc Dis. 2019;62:384–389 [DOI] [PubMed] [Google Scholar]
- 22.Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis. 2018;277:483–492 [DOI] [PubMed] [Google Scholar]
- 23.Sunil B, Ashraf AP. Dyslipidemia in pediatric type 2 diabetes mellitus. Curr Diab Rep. 2020;20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogachkov YY, Chen L, Le Master E, Fancher IS, Zhao Y, Aguilar V, Oh MJ, Wary KK, DiPietro LA, Levitan I. Ldl induces cholesterol loading and inhibits endothelial proliferation and angiogenesis in matrigels: Correlation with impaired angiogenesis during wound healing. Am J Physiol Cell Physiol. 2020;318:C762–C776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Inward rectifier potassium (kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol. 2016;594:3271–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh DK, Shentu T-P, Enkvetchakul D, Levitan I. Cholesterol regulates prokaryotic kir channel by direct binding to channel protein. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808:2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbera N, Ayee MAA, Akpa BS, Levitan I. Molecular dynamics simulations of kir2.2 interactions with an ensemble of cholesterol molecules. Biophys J. 2018;115:1264–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenhouse-Dantsker A, Noskov S, Durdagi S, Logothetis DE, Levitan I. Identification of novel cholesterol-binding regions in kir2 channels. Journal of Biological Chemistry. 2013;288:31154–31164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han H, Rosenhouse-Dantsker A, Gnanasambandam R, Epshtein Y, Chen Z, Sachs F, Minshall RD, Levitan I. Silencing of kir2 channels by caveolin-1: Cross-talk with cholesterol. J Physiol. 2014;592:4025–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannattasio C, Mangoni AA, Failla M, Carugo S, Stella ML, Stefanoni P, Grassi G, Vergani C, Mancia G. Impaired radial artery compliance in normotensive subjects with familial hypercholesterolemia. Atherosclerosis. 1996;124:249–260 [DOI] [PubMed] [Google Scholar]
- 31.Hayoz D, Weber R, Rutschmann B, Darioli R, Burnier M, Waeber B, Brunner HR. Postischemic blood flow response in hypercholesterolemic patients. Hypertension. 1995;26:497–502 [DOI] [PubMed] [Google Scholar]
- 32.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart. 2003;89:1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvany MJ. Resistance vessel structure and the pathogenesis of hypertension. J Hypertens Suppl. 1993;11:S7–12 [PubMed] [Google Scholar]
- 34.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2012;32:2531–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoud AM, Hwang CL, Szczurek MR, Bian JT, Ranieri C, Gutterman DD, Phillips SA. Low-fat diet designed for weight loss but not weight maintenance improves nitric oxide-dependent arteriolar vasodilation in obese adults. Nutrients. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paniagua OA, Bryant MB, Panza JA. Role of endothelial nitric oxide in shear stress-induced vasodilation of human microvasculature: Diminished activity in hypertensive and hypercholesterolemic patients. Circulation. 2001;103:1752–1758 [DOI] [PubMed] [Google Scholar]
- 37.Steinberg D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217 [DOI] [PubMed] [Google Scholar]
- 38.Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and ion channels. Subcell Biochem. 2010;51:509–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.