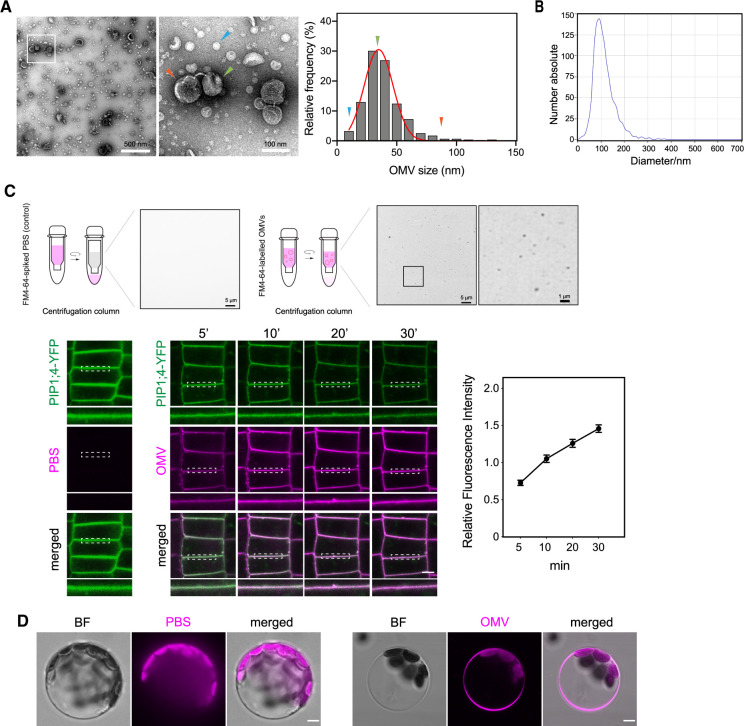
Figure 1.
Bacterial OMVs’ morphology and direct insertion of OMVs into plant PM. A, TEM micrographs of OMVs extracted from cell-free Xcc culture supernatant from NYG culture (bar, 500 nm in the left and 100 nm in the right). Arrowheads indicate several representative sizes of OMVs observed in the electron micrographs. The frequency distribution of OMV diameter was curve fitted using Gaussian distribution with GraphPad Prism 8.0. B, Diameter of Xcc OMVs measured by NTA. C, Labeling of Xcc OMVs with FM4–64 dye using centrifugal filter (bars, 5 µm and 1 µm) and fusion of FM4–64-labeled Xcc OMV to plant PM. Super-resolution of FM4–64-labeled OMVs was acquired on a Nikon Ti2 system equipped with Live-SR structured illumination microscopy. Xcc OMVs was labeled with FM4–64 dye, then applied onto Arabidopsis YFP-PIP1;4 seedling roots. Seedlings were mounted immediately, and the confocal images of root cells were taken at indicated time-points up to 30 min after incubation. Insets beneath each image are regions marked by white dashed boxes (bar, 5 µm). Relative fluorescence intensity of FM4–64 (OMV) signal on the PM over time was measured by Fiji and normalized to the intensity of the corresponding ROIs in the Green (YFP-PIP1;4) channel. In addition, PBS buffer was spiked with FM4–64 dye, subjected to centrifugation on a centrifugal column and the left-over buffer on the column was used to apply onto YFP-PIP1;4 plants and imaged as control. D, Integration of FM4–64 labeled OMVs into the PM of Arabidopsis Col-0 protoplast. FM4–64-labeled Xcc OMVs were applied on leaf protoplasts for 30 min (bar, 5 µm) prior to imaging.