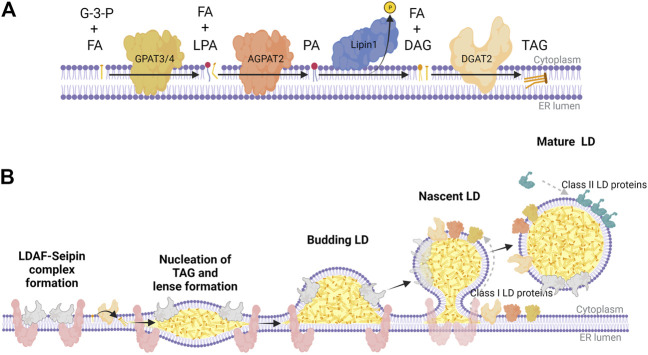
FIGURE 2.
The Kennedy pathway for TAG synthesis and LD biogenesis in mammalian cells. (A) Biosynthetic enzymes of the Kennedy pathway act sequentially to synthesize triacylglycerol (TAG) at the ER membrane; GPAT3/4 synthesizes lysophosphatidic acid (LPA) from glycerol 3-phosphate (G-3-P) and fatty acids (FA), AGPAT2 synthesizes phosphatidic acid (PA) from LPA and FA, Lipin-1 hydrolyzes PA to diacylglycerol (DAG), and DGAT2 catalyzes the final acylation to form TAG. (B) TAG nucleates between the two leaflets of the ER membrane bilayer, which is partly facilitated by a complex of LDAF and seipin at distinct domains throughout the ER. These points of TAG nucleation develop into lens-like structures that proceed to bud into the cytoplasm as a budding LD coated with LDAF as it dissociates from seipin. As seipin funnels TAG and DAG into nascent LDs, lipid biosynthetic enzymes (class I LD proteins) like GPAT3/4, AGPAT2, and DGAT2 transfer across membrane bridges to the surface monolayer, further facilitating the maturation of LDs. Once the mature LD separates from the ER, it recruits class II LD proteins like perilipin-2/3, which coat the surface to regulate access of LDs to lipases and autophagy proteins. This graphic was created with Biorender.com.