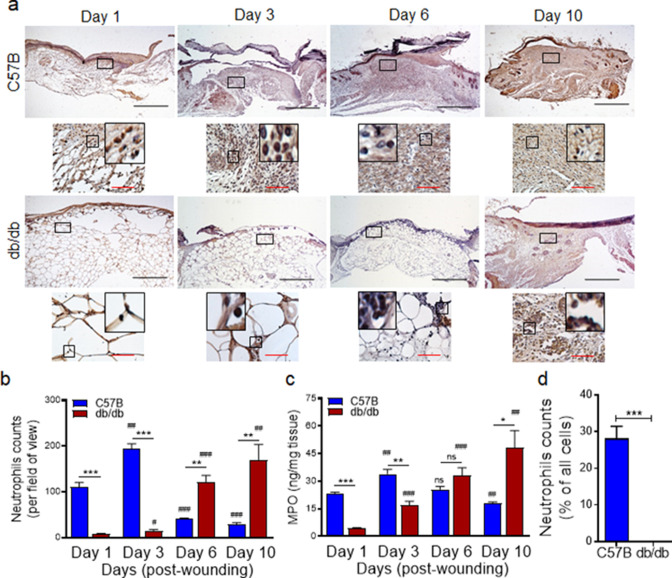
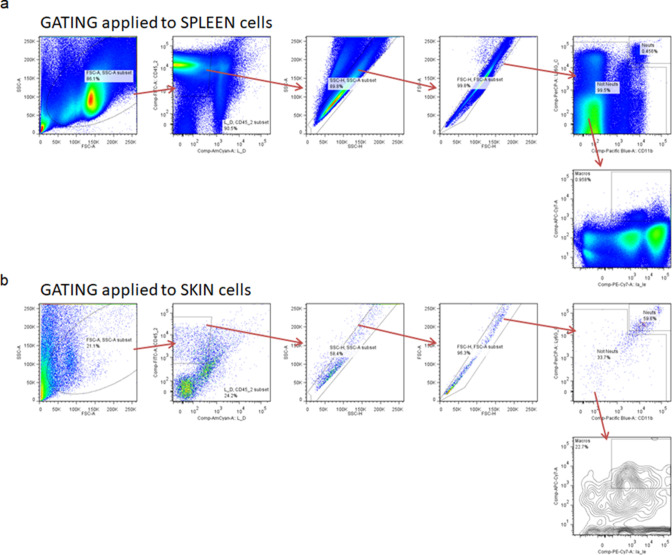
Figure 1. Neutrophil response is delayed in infected diabetic wound tissue.
Normal (C57BL/6) and diabetic (db/db) wounds were infected with PA103 (1000 CFU/wound). (a–b) Wound tissues were harvested at indicated timepoints post-infection and assessed for neutrophil contents by histological analysis using anti-Ly6G antibody. (a) Representative regions from underneath the wounds extending in the dermis are shown at ×40 and ×400 magnification (top and bottom, respectively). A representative magnified region is also inserted in the ×400 magnification images. Black scale bar = 500 µm for ×40 magnification and red scale bar = 50 µm for ×400 magnification. (b) The corresponding data were plotted as the Mean ± SEM. (c) Wounds at indicated timepoints were assessed for their MPO contents by ELISA and the tabulated data are shown as the Mean ± SEM. (d) Day 1 infected wound tissues of C57BL/6 and db/db were evaluated for their neutrophil contents by flow cytometry. Corresponding data were plotted as the Mean ± SEM. (N = 4; ns = not significant, *p < 0.05; **p < 0.01; ***p < 0.001 – are comparisons made between C57BL/6 and db/db at indicated timepoints; or #p < 0.05; ##p < 0.01; ###p < 0.001 are comparisons made within each group to day one values, respectively. Statistical analyses between groups were conducted by One-way ANOVA with additional post hoc testing, and pair-wise comparisons between groups were performed or by unpaired Student’s t-test).
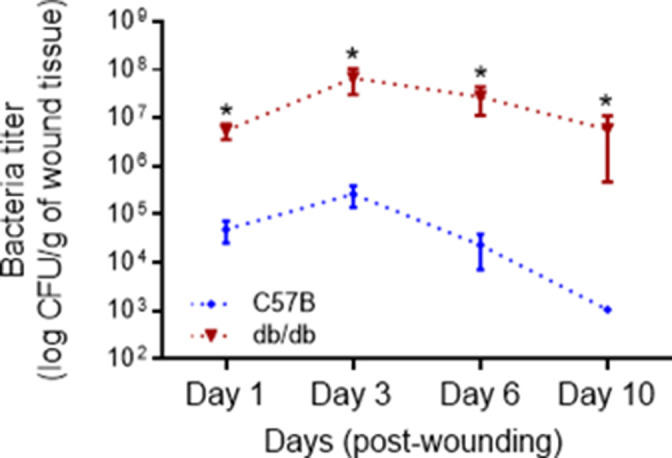
Figure 1—figure supplement 1. Diabetic wound is vulnerable to increased infection with Pseudomonas aeruginosa.