Abstract
Aims
An improved understanding of the pathophysiology of trastuzumab-mediated cardiotoxicity is required to improve outcomes of patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer. We aimed to characterize the cardiac and cardiometabolic phenotype of trastuzumab-mediated toxicity and potential interactions with cardiac pharmacotherapy.
Methods and results
This study was an analysis of serial magnetic resonance imaging (MRI) and circulating biomarker data acquired from patients with HER2-positive early-stage breast cancer participating in a randomized-controlled clinical trial for the pharmaco-prevention of trastuzumab-associated cardiotoxicity. Circulating biomarkers (B-type natriuretic peptide, troponin I, MMP-2 and -9, GDF-15, neuregulin-1, and IGF-1) and MRI of cardiac structure and function and abdominal fat distribution were acquired prior to trastuzumab, post-cycle 4 and post-cycle 17. Ninety-four participants (51 ± 8 years) completed the study with 30 on placebo, 33 on perindopril, and 31 on bisoprolol. Post-cycle 4, global longitudinal strain deteriorated from baseline in both placebo (+2.0 ± 2.7%, P = 0.002) and perindopril (+0.9 ± 2.5%, P = 0.04), but not with bisoprolol (−0.2 ± 2.1%, P = 0.55). In all groups combined, extracellular volume fraction and GDF-15 increased post-cycle 4 (+1.3 ± 4.4%, P = 0.004; +130 ± 150%, P ≤ 0.001, respectively). However, no significant change in troponin I was detected throughout trastuzumab. In all groups combined, visceral and intermuscular fat volume increased post-cycle 4 (+7 ± 17%, P = 0.02, +8 ± 23%, P = 0.02, respectively), while muscle volume and IGF-1 decreased from post-cycle 4 to 17 (−2 ± 10%, P = 0.008, -18 ± 28%, P < 0.001, respectively).
Conclusion
Trastuzumab results in impaired cardiac function and early myocardial inflammation. Trastuzumab is also associated with deleterious changes to the cardiometabolic phenotype which may contribute to the increased cardiovascular risk in this population.
Keywords: Trastuzumab, Cardiotoxicity, Body composition, Magnetic resonance imaging
Introduction
Human epidermal growth factor receptor 2 (HER2) is overexpressed in ∼20% of breast cancers. The anti-HER2 agent trastuzumab reduces risk of disease progression or death by 40%.1 However, HER2 isoforms are also expressed in cardiomyocytes and appear to play a critical role in regulating cardiac structure and function through neuregulin-1 signalling.2 As a result, trastuzumab is also associated with a dose-independent two-fold increased risk of left ventricular (LV) dysfunction and a five- to seven-fold increased risk of heart failure.1,3 Furthermore, ∼22% of patients receiving trastuzumab will experience interruptions or discontinuation due to cardiac events.4 However, the pathophysiology of trastuzumab cardiotoxicity has not been well elucidated and requires further characterization to enable maintenance of cancer treatment efficacy and minimization of cardiovascular risk.
Circulating biomarkers have utility for the early detection of anthracyclines and/or trastuzumab-mediated cardiotoxicity5 and also have the potential to elucidate involved mechanisms. For example, high-sensitivity troponin, a marker of myocardial injury, is elevated following anthracycline chemotherapy for breast cancer,5–8 but its response to trastuzumab is less characterized. Similarly, biomarkers related to cardiac energetics, metabolism, and extracellular matrix could also provide mechanistic insight into trastuzumab-mediated cardiotoxicity. Cardiac magnetic resonance imaging (MRI) is the gold standard imaging modality for cardiac structure and function and can also assess myocardial fibrosis and/or inflammation through T1 mapping. Magnetic resonance imaging can also be used to quantify abdominal fat and muscle distribution changes. On computed tomography, these metrics predict chemotherapy tolerability and incident cardiovascular disease in patients with breast cancer.9,10 However, the longitudinal effects of chemotherapy and trastuzumab on abdominal composition are unknown and could plausibly play an important role in cardiometabolic risk. To date, no studies have comprehensively evaluated serial changes in cardiac structure and function as well as body composition during trastuzumab therapy.
We previously conducted a randomized-controlled trial, MANTICORE 101-Breast, of bisoprolol, a beta-blocker, or perindopril, an angiotensin-converting enzyme inhibitor (ACEi), for the prevention of trastuzumab-mediated cardiotoxicity and found that these agents attenuated declines in cardiac MRI-derived LV ejection fraction (EF) compared with placebo.11 In this secondary analysis of the MANTICORE trial, we aim to further characterize the longitudinal cardiac and cardiometabolic effects of trastuzumab via imaging and circulating biomarkers and the impact of pharmaco-intervention.
Methods
Design and ethics
The study was a double-blind, three-arm, parallel group, randomized, placebo-controlled trial (clinicaltrials.gov: NCT01016886). Participants were randomized 1:1:1 to bisoprolol, perindopril, or placebo during trastuzumab therapy (∼12 months). This trial complied with the Declaration of Helsinki and obtained approval from institutional health research ethics boards. All participants provided written informed consent. The protocol has been previously reported.12
Participants
Adults (>18 years) diagnosed with HER2-positive early-stage (I-IIIA) breast cancer and scheduled to receive adjuvant trastuzumab were recruited from two tertiary cancer hospitals in Edmonton and Winnipeg, Canada. Exclusion criteria included baseline LVEF <50%, history of heart failure, myocardial infarction or cardiomyopathy, uncontrolled hypertension, contraindications to study drugs or MRI, concurrent treatment with ACEi, beta-blockers, or angiotensin receptor antagonist, prior thoracic radiation or chemotherapy, or estimated glomerular filtration rate <30 mL/min/1.73 m2.
Outcomes
Magnetic resonance imaging
Comprehensive cardiac MRI scans were performed on 1.5 T system (Siemens Healthcare, Erlangen, Germany) at three time points: (i) prior to trastuzumab therapy (baseline); (ii) after four trastuzumab treatment cycles (∼3 months from baseline); and (iii) at completion of trastuzumab therapy (typically 17 cycles in total, ∼12 months from baseline). Steady-state free precession cine imaging was acquired at end-inspiration with retrospective electrocardiographic (ECG) gating and reconstruction to 30 phases. Left ventricular, right ventricular (RV), and left atrium (LA) volume analysis was performed by an experienced cardiac MRI interpreter blinded to treatment assignment on commercial software (Syngo Argus; Siemens Healthcare, Erlangen, Germany). Left ventricular volumes and mass and RV volumes were calculated from a contiguous short-axis stack of cines using a method of disks approach. Left atrium volumes were estimated using a modified area-length biplane method. Volumes and masses at all time points were indexed to the baseline body surface area. LV global longitudinal strain (GLS) was analysed as the average endocardial longitudinal strain of the two-, three-, and four-chamber long-axis views using custom in-house software (MATLAB, The Mathworks, Natick, MA, USA). T1 mapping was acquired from a basal short-axis slice using saturation-recovery single-shot acquisition pulse sequence prior to and 20 min following intravenous administration of gadopentetate dimeglumine (0.15 mmol/kg; Magnevist, Bayer Schering, Germany); septal extracellular volume (ECV) was calculated using measured haematocrit.13
In the same scan, a 20-slice, single-shot, fast-spin-echo acquisition was used to generate high fat–water contrast images of the abdomen for segmentation of fat and muscle. Images were obtained prior to the cardiac scan using a body matrix coil and the relevant spine array coils. An axial stack with 6 mm slice thickness (no gaps) and 1.04 mm2 in-plane resolution was acquired with a scan time of ≤5 min. Five slices centred around the third lumbar vertebral body were analysed. The sum of the volumes of muscle, intermuscular, visceral, and subcutaneous fat for the five slices was measured using custom semi-automated software to identify the boundaries of subcutaneous fat, muscle, and visceral fat. Bones and organs were removed from the visceral fat pool. Interrater analysis reliability for these measurements in 10 random subjects was excellent, with intraclass correlation coefficients: subcutaneous fat, visceral fat, and muscle = 0.99 each; intermuscular fat = 0.77. Muscle and fat volumes from a cross-section at the third lumbar vertebra have been shown to be accurate relative to cadaver measurements and representative of whole-body composition.14,15
Circulating biomarkers
Venipunctures were performed prior to each cardiac MRI scan. Blood samples were centrifuged for 15 min at 1000 g within 30 min of collection and cryostored at −80°C for future batch analysis. We analysed circulating biomarkers associated with myocardial injury [high-sensitivity troponin I, B-type natriuretic peptide (BNP)], inflammation (GDF-15), or matrix remodelling (MMP-2, MMP-9), as well as primary agonists of the tyrosine receptor growth factor axis (neuregulin-1, IGF-1).
B-type natriuretic peptide and high-sensitivity troponin I were measured using a two-site alkaline phosphatase-conjugated chemiluminescent immunoassay by an automated Beckman Coulter DxI 800 analyser. For the BNP assay, the detection limit is 1 ng/L with minimal cross-reactivity with other related peptides. For the troponin assay, the Beckman Coulter recommended 99th percentile was 11.6 ng/L for females, coefficient of variation of <10% at 5.6 ng/L, limit of blank of 0.27 ng/L, and limit of detection of 0.90 ng/L. Plasma GDF-15, IGF-1, neuregulin-1, MMP-2 and -9 levels were determined using commercially available human enzyme-linked immunosorbent assay kits from R&D systems (DGD150, DG100, DY377, MMP200, and DMP900, respectively, Minneapolis, MN, USA) following manufacturer instructions. Absorbance was measured at 450 nm and corrected against 540 nm using a SpectraMax microplate reader (Soft Max®Pro 5). These kits show negligible cross-reactivity (<0.5%) and high sensitivity (detection limits by manufacturer: GDF-15, 4.39 pg/mL; IGF-1, 0.056 ng/mL; MMP-2, 0.082 ng/mL; MMP-9, 0.156 ng/mL).
Statistical analysis
To characterize the cardiac phenotype of trastuzumab-mediated cardiotoxicity and the mediating effects of pharmacotherapy, two statistical approaches were used. First, temporal changes in cardiac MRI measures and circulating biomarkers were analysed for the groups independently using generalized linear mixed models. Participant was used as a random effect and time as a fixed and repeated effect. The distribution and link function that produced normality of model residuals (determined used QQ plots) was used. When normality was not achieved with any combination of distribution and link function, the model with the best fit, determined by comparing Akaike Corrected Information Criterion between models was used. Significant time main effects were investigated using contrasts between each time point. When a histogram indicated significant deviation from a normal distribution, circulating biomarkers were log-transformed and are reported as median (interquartile range). For body composition parameters, all groups were combined for this analysis due to lack of rationale for effect of bisoprolol or perindopril on these metrics. Second, to understand intergroup differences in the cardiac MRI and biomarkers, the magnitude of the change from baseline to post-cycle 4 and post-cycle 17 in all variables were compared between groups using analysis of covariance, with the baseline values as a covariate. Baseline values of each variable were also compared across groups using a one-way analysis of variance with post hoc pairwise comparisons by either Tukey’s or Games–Howell tests, depending on equality of variances. To determine the potential influence of pre-trastuzumab receipt of anthracycline treatment, subgroup analysis was performed comparing anthracycline vs. non-anthracycline regimens at each time point using independent t-tests. A P-value of ≤0.05 was used to denote statistical significance. SPSS version 26.0 (IBM Corp, Armonk, NY, USA) was used for all analyses.
Results
Clinical data
Ninety-four participants completed the study (n = 30, placebo; n = 33, perindopril; n = 31, bisoprolol). Patients received a regimen of docetaxel, carboplatin, and trastuzumab (TCH) every 3 weeks for 6 cycles (77%), or 5-fluorouracil, epirubicin and cyclophosphamide (FEC) every 3 weeks for 3 cycles, followed by docetaxel and trastuzumab every 3 weeks for 3 cycles (DH, 23%). In both regimens, trastuzumab was given every 3 weeks concurrent with docetaxel and then alone for a total of 17 cycles. Forty-one per cent also received left-sided radiotherapy. The groups did not differ in cancer pathology, treatment, or baseline demographic or cardiovascular profiles (Table 1). The mean dose received was 7 ± 2 mg/day for perindopril and 8 ± 3 mg/day for bisoprolol and there were no study drug withdrawals. As previously reported, baseline heart and blood pressure were similar between groups, while bisoprolol significantly reduced heart rate relative to placebo and perindopril, and perindopril and bisoprolol reduced systolic and diastolic blood pressure relative to placebo.11
Table 1.
Participant characteristics
| Placebo | Bisoprolol | Perindopril | P-value | |
|---|---|---|---|---|
| n = 30 | n = 31 | n = 33 | ||
| Age (years), mean ± SD | 51 ± 7 | 53 ± 10 | 50 ± 8 | 0.41 |
| Body surface area (m2), mean ± SD | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 | 0.13 |
| Pre-trastuzumab receipt of anthracyclines, n (%) | 7 (23%) | 4 (12%) | 11 (35%) | 0.17 |
| Cardiovascular risk factors | ||||
| Hypertension, n (%) | 2 (7%) | 2 (6%) | 0 | 0.36 |
| Type II diabetes, n (%) | 0 | 3 (10%) | 1 (3%) | 0.16 |
| Smoking status, n (%) | 0.20 | |||
| Current | 3 (10%) | 3 (10%) | 2 (6%) | |
| Former | 12 (40%) | 7 (23%) | 12 (42%) | |
| Dyslipidaemia | 0 | 1 (3%) | 1 (3%) | 0.63 |
Cardiac magnetic resonance imaging
Data for LV volumes, EF, and mass and cardiotoxicity incidence have been previously reported.11 Patients who had received FEC (n = 22) prior to the baseline assessment had lower LV function compared with patients scheduled to receive TCH (n = 72) (LVEF: 60 ± 5% vs. 63 ± 4%, P = 0.02; GLS: −18.1 ± 2.8% vs. −19.9 ± 2.4%, P = 0.005). However, these regimen-based differences had dissipated by post-cycle 4 and 17. LV end-systolic volume index increased significantly and to a similar extent in all three intervention groups but declines in LVEF post-cycle 4 and 17 were attenuated in the bisoprolol and perindopril groups by increased end-diastolic volume index (Table 2).
Table 2.
Cardiac magnetic resonance imaging data
| Placebo | Bisoprolol | Perindopril | Baseline (ANOVA P-value) | |
|---|---|---|---|---|
| (n = 30) | (n = 31) | (n = 33) | ||
| LV end-diastolic volume (mL/m2) | ||||
| Baseline | 76 ± 13 | 69 ± 10 | 67 ± 14a | 0.01 |
| Post-cycle 4 | 76 ± 9 | 75 ± 11b | 70 ± 15b | |
| Post-cycle 17 | 79 ± 12 | 76 ± 14b | 74 ± 16b | |
| LV end-systolic volume (mL/m2) | ||||
| Baseline | 30 ± 7 | 26 ± 5a | 25 ± 6a | 0.01 |
| Post-cycle 4 | 35 ± 7b | 31 ± 5b | 29 ± 8b | |
| Post-cycle 17 | 35 ± 8b | 30 ± 6b | 30 ± 8b | |
| LV ejection fraction (%) | ||||
| Baseline | 61 ± 5 | 62 ± 4 | 62 ± 5 | 0.55 |
| Post-cycle 4 | 54 ± 5b | 59 ± 4 | 59 ± 6 | |
| Post-cycle 17 | 56 ± 4b | 61 ± 4 | 59 ± 6 | |
| LV mass (g/m2) | ||||
| Baseline | 53 ± 8 | 51 ± 7 | 52 ± 7 | 0.63 |
| Post-cycle 4 | 55 ± 8 | 52 ± 8 | 52 ± 7 | |
| Post-cycle 17 | 53 ± 8 | 52 ± 6 | 52 ± 8 | |
| LV global longitudinal strain (%) | ||||
| Baseline | −20.1 ± 2.5 | −19.4 ± 2.6 | −19.0 ± 2.5 | 0.24 |
| Post-cycle 4 | −18.1 ± 2.7b | −19.6 ± 2.3 | −18.1 ± 2.4b | |
| Post-cycle 17 | −18.8 ± 2.5b | −20.4 ± 2.0b | −18.9 ± 2.2 | |
| RV end-diastolic volume (mL/m2) | ||||
| Baseline | 71 ± 16 | 63 ± 12 | 65 ± 12 | 0.07 |
| Post-cycle 4 | 71 ± 13 | 69 ± 12b | 69 ± 14b | |
| Post-cycle 17 | 74 ± 14 | 71 ± 14b | 71 ± 17b | |
| RV end-systolic volume (mL/m2) | ||||
| Baseline | 27 ± 9 | 24 ± 6 | 26 ± 7 | 0.15 |
| Post-cycle 4 | 32 ± 8b | 27 ± 7b | 30 ± 6b | |
| Post-cycle 17 | 31 ± 9b | 28 ± 8b | 29 ± 8b | |
| RV ejection fraction (%) | ||||
| Baseline | 61 ± 7 | 62 ± 6 | 60 ± 6 | 0.31 |
| Post-cycle 4 | 55 ± 6b | 60 ± 6 | 57 ± 6b | |
| Post-cycle 17 | 59 ± 6b | 61 ± 8 | 60 ± 6 | |
| Left atrial volume (mL/m2) | ||||
| Baseline | 44 ± 9 | 36 ± 9a | 37 ± 10a | <0.01 |
| Post-cycle 4 | 45 ± 10 | 44 ± 9b | 42 ± 12b | |
| Post-cycle 17 | 46 ± 14 | 45 ± 11b | 43 ± 11b |
Data are mean ± standard deviation.
ACE, angiotensin-converting enzyme; LV, left ventricular; RV, right ventricular; missing data: global longitudinal strain, n = 3; native T1 time, n = 36; extracellular volume, n = 39.
Different than placebo (P < 0.05).
Different than baseline (P < 0.05).
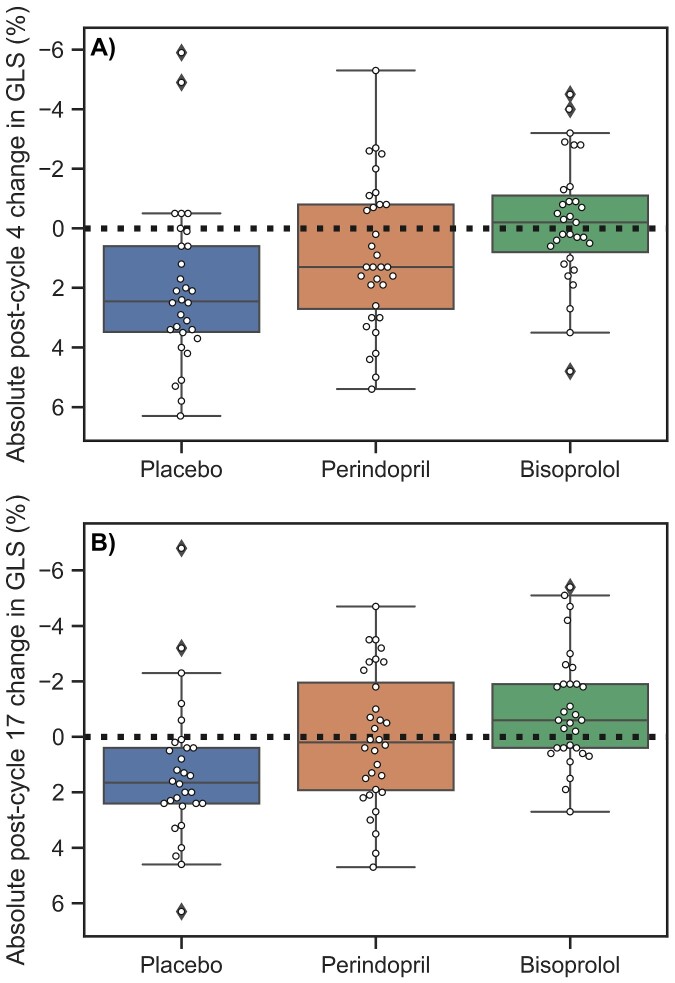
Post-cycle 4, LV GLS deteriorated from baseline in both placebo (+2.0 ± 2.7%) and perindopril (+0.9 ± 2.5%) groups but not with bisoprolol (−0.2 ± 2.1%, P < 0.01 compared with other groups, Table 2, Figure 1). Post-cycle 17, LV GLS remained reduced relative to baseline in the placebo group (+1.3 ± 2.5%), recovered in the perindopril group (+0.1 ± 2.4%), and was improved in the bisoprolol group (−1.0 ± 2.0, P < 0.01 compared with other groups, Figure 1). In each group, RV volumes changed in parallel to LV volumes (Table 2). Post-cycle 4, RVEF was decreased from baseline in placebo (−6 ± 6%) and perindopril (−3 ± 7%), but not bisoprolol (−2 ± 6%), but only bisoprolol differed from placebo (P < 0.01). Similar to both ventricles, LA volume did not change in the placebo group but was increased relative to baseline post-cycle 4 and 17 in bisoprolol (+7 ± 9 and +5 ± 8 mL/m2, respectively) and perindopril (+5 ± 8 and +7 ± 12 mL/m2, respectively, Table 2).
Figure 1.
Boxplots and individual data for absolute changes in global longitudinal strain between treatment groups after (A) cycle 4 (∼3 months) and (B) cycle 17 (∼12 months). Box shows interquartile range and median (middle line), with whiskers extending to the rest of the distribution except for outliers (defined as 1.5*interquartile range, indicated by diamonds). The dotted lines indicate no change.
T1 and ECV data were not available in 14% of scans and therefore analysis was performed for all patients combined. Cancer regimen had no effect on observed native T1 and ECV at any time point. In all patients, ECV fraction increased significantly from baseline to post-cycle 4 (22.9 ± 3.3 to 24.3 ± 3.4%, P = 0.004) but native T1 did not change (1200 ± 41 to 1207 ± 46, P = 0.28). By post-cycle 17, ECV fraction had returned to baseline (22.4 ± 3.5%, P = 0.36) and native T1 was lower than baseline (1184 ± 46 ms, P = 0.02).
Abdominal magnetic resonance imaging—body composition
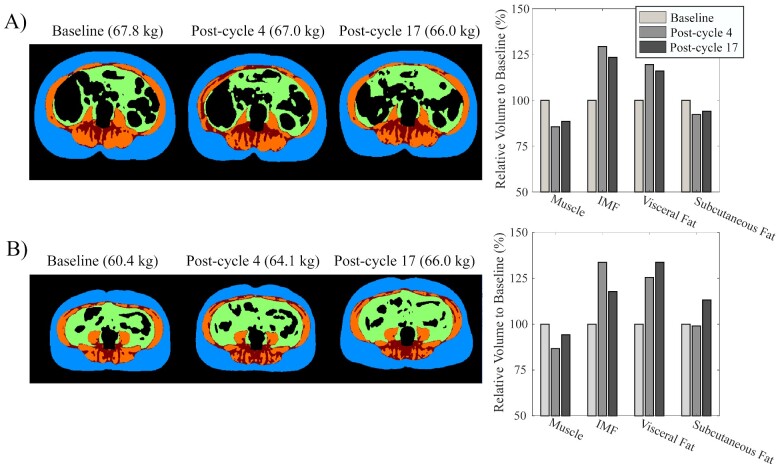
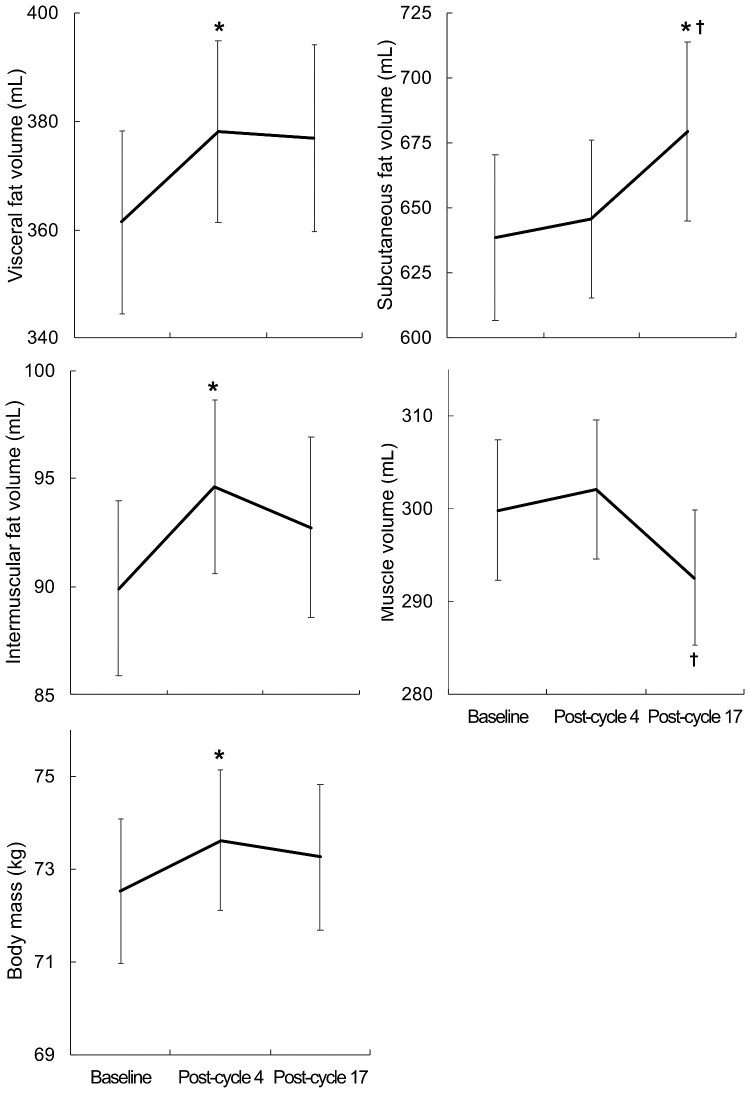
Follow-up body composition imaging was not available in 8 (9%) participants and subcutaneous fat was excluded in 17% of cases as it was outside the field of view of the scanner; otherwise image quality allowed differentiation of fat and skeletal muscle (Figure 2, Supplementary material online, File). Body mass and all fat depots were significantly higher or trended in that direction in those who had received FEC at baseline, post-cycle 4 and 17 (mass: P = 0.008, 0.007, 0.03; subcutaneous: P = 0.05, 0.02, 0.07; visceral: P = 0.009, 0.06, 0.12; inter-muscular: P = 0.04, 0.009, 0.03, respectively); while muscle volume did not differ. In the overall cohort, there was an early increase at post-cycle 4 in body mass (+2 ± 5%, P = 0.008), visceral (+7 ± 17%, P = 0.02), and inter-muscular fat (+8 ± 23%, P = 0.02; Figure 3). Between post-cycle 4 and 17, muscle volume decreased (−2 ± 10%, P = 0.008), and subcutaneous fat volume increased (+6 ± 23%, P = 0.02; Figure 3). Over the 12 months of trastuzumab therapy, the relative increase in abdominal adiposity was disproportionate to the increase in overall body weight (1 ± 8%): subcutaneous fat (6 ± 23%), visceral fat (4 ± 19%), and inter-muscular fat (7 ± 25%). Skeletal muscle decreased −2 ± 10% over the same time frame.
Figure 2.
Examples of body composition: subcutaneous fat (blue), visceral fat (green), intermuscular fat (red), and muscle (orange) across the three time points in (A) a representative case with body weight loss across trastuzumab concurrent to muscle volume loss and fat volume elevation; and (B) a representative case of weight gain across trastuzumab concurrent to muscle volume loss and fat volume elevation. IMF, inter-muscular fat.
Figure 3.
Body composition changes over time for all groups combined. *Difference from baseline (P ≤ 0.05). †Difference from post-cycle 4 (P ≤ 0.05).
Biomarkers
At baseline, patients who had received FEC had significantly elevated circulating biomarkers of myocardial injury and inflammation compared with those scheduled to received TCH, high-sensitivity troponin [8.0 (5.9–12.3) vs. 2.1 (1.6–3.0) ng/L, P < 0.001], GDF-15 [616 (514–888) vs. 494 (384–597) ng/mL, P = 0.001], and MMP-2 (234 ± 37 vs. 191 ± 45 ng/mL, P < 0.001), respectively. High-sensitivity troponin remained higher in FEC at post-cycle 4 [5.0 (3.9–7.5) vs. 2.4 (1.9–3.5) ng/L, P < 0.001] and post-cycle 17 [3.2 (2.4–4.0) vs. 2.5 (1.6–3.5) ng/L, P = 0.02].
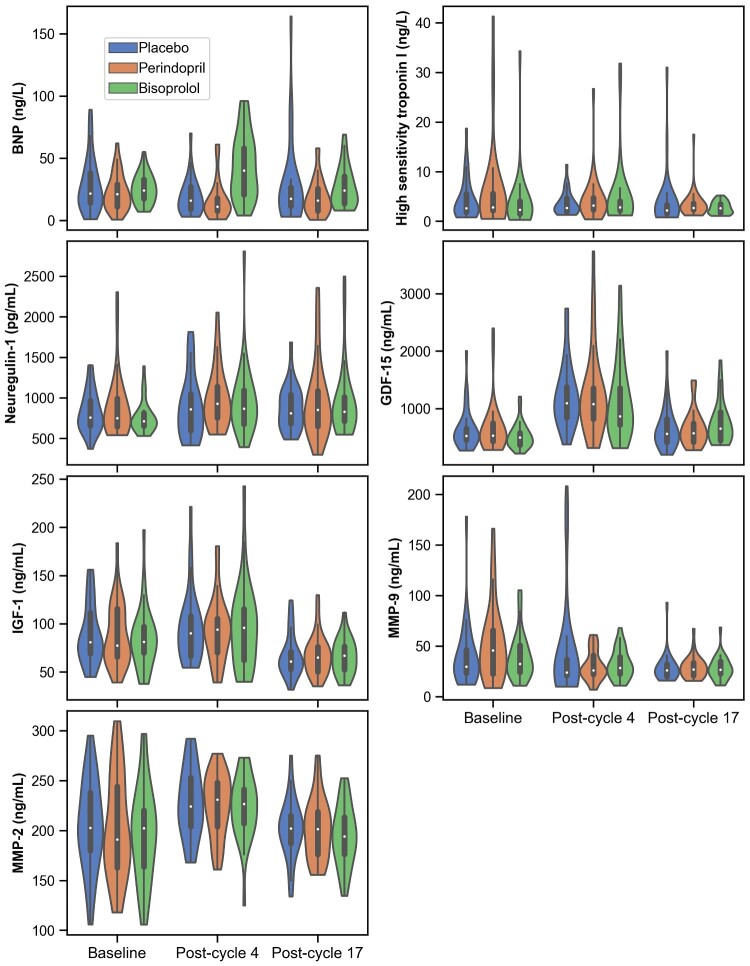
Regarding temporal changes in biomarkers by pharmaco-intervention, there were no between-group differences for magnitude of change other than for BNP which transiently increased in bisoprolol treated patients and transiently decreased in those receiving placebo or perindopril (Figure 4). Also, only in the bisoprolol group, neuregulin-1 increased from baseline at post-cycle 4 [713 (653–814) pg/mL to 869 (674–1094) pg/mL, P = 0.002] and post-cycle 17 [829 (718–1010) pg/mL, P = 0.03].
Figure 4.
Violin plots of distribution of circulating biomarkers by treatment group over time. Inside box shows interquartile range and median (white dot), with whiskers extending to the rest of the distribution except for outliers (>1.5 * interquartile range). The range of the plot includes all of the observed data. The coloured shape outside of the lines is a kernel density estimation to show the distribution of the data.
The changes over time for the remaining biomarkers were very similar within each group (Figure 4). IGF-1 was markedly decreased at post-cycle 17 (all groups combined: −18 ± 2% from baseline, P ≤ 0.001 within each group, Figure 4). GDF-15 transiently increased substantially from baseline to post-cycle 4 (+130 ± 150% from baseline, P ≤ 0.002 within each group, Figure 4). Similarly, MMP-2 increased transiently in all patients. Notably, high-sensitivity troponin I did not increase post-cycle 4 or 17 in any group.
Discussion
This secondary analysis of the MANTICORE trial contributes several new findings regarding temporal changes in the cardiac phenotype and body composition during trastuzumab therapy. We found that trastuzumab-mediated cardiotoxicity is characterized by concurrent LV and RV dysfunction and reversible myocardial inflammation and oedema. While bisoprolol and perindopril preserved biventricular function relative to placebo, they had minimal effects on circulating cardiac biomarkers. Our non-cardiac imaging and circulating biomarkers results suggest unfavourable body composition and growth factor adaptations during trastuzumab that could contribute to increased cardiovascular risk in breast cancer survivors independent of direct cardiac effects.
Effect of anthracyclines prior to trastuzumab
Those patients pre-treated with anthracyclines (FEC) prior to trastuzumab had reduced cardiac function (LVEF, GLS) and increased circulating biomarkers of injury (high-sensitivity troponin), inflammation (GDF-15), and remodelling (MMP-2), and greater abdominal fat compared with chemotherapy-naïve patients at our pre-trastuzumab baseline assessment. While the differences in GDF-15, MMP-2, or LV function did not persist throughout trastuzumab, high-sensitivity troponin I and abdominal fat remained higher post-cycle 4 and 17 in those who had received initial sequential anthracycline therapy. While elevated troponin has been mechanistically linked to anthracycline-related myocardial injury, further study is required to better characterize the impact of anthracycline therapy on the cardiometabolic profile.
Changes in cardiac phenotype
We found that trastuzumab-related RV dysfunction and remodelling occurs in parallel to changes of the LV. In an observational study of 41 patients receiving trastuzumab ± anthracyclines, biventricular function also decreased at 6 months and subsequently recovered to baseline by 18 months.16 We extend these findings by showing that perindopril and bisoprolol had protective effects on depression of LV GLS post-cycle 17.
In all groups, we observed an increase in GDF-15, MMP-2, and ECV fraction from baseline to post-cycle 4 followed by a return to baseline post-cycle 17 without concurrent changes in high-sensitivity troponin I. GDF-15 is a stress-induced cytokine released from vascular smooth muscle cells and cardiomyocytes in response to injury, anoxia, and inflammation,17 while MMP-2 is involved in the proteolysis of several myocardial proteins, leading to reduced contractility and cardiac dysfunction.18 These findings suggest that trastuzumab induces early myocardial inflammation and oedema, in line with previous preclinical reports of mononuclear cell infiltration and myocardial edema19,20 and clinical reports of myocardial oedema on T2-weighted imaging after trastuzumab exposure.21 However, we found that myocardial oedema is transient, with no discernable relationship to changes in cardiac function.
In contrast to prior studies of anthracycline-mediated cardiotoxicity, we found no increase in high-sensitivity troponin during trastuzumab therapy.5–8 A similar finding was reported in 71 patients with breast cancer undergoing serial testing during non-anthracycline, trastuzumab-based treatment.22 Therefore, cardiomyocyte injury does not appear to play a critical role in trastuzumab-mediated cardiotoxicity and, consequently, expert consensus recommendation of measuring troponin every 3 months during trastuzumab therapy23 may not provide added information to imaging unless patients also receive anthracyclines.
Neuregulin-1 is a paracrine growth factor that is released from cardiac and vascular endothelial cells in response to physiologic stress and binds to nearby ErbB receptors to promote adaptation of cardiac structure and function.24 As trastuzumab disrupts neuregulin-1/ErbB signalling, the small drop in neuregulin-1 reported by Geisberg et al.24 with anthracycline or trastuzumab (37% HER2-positive) therapy was suggested to represent a loss in cardio-reparative capacity. Our finding of bisoprolol increasing neuregulin-1 by post-cycle 4 with maintenance to post-cycle 17 suggests that potentially some of the protection by bisoprolol was mediated by enhancement of endogenous neuregulin-1 levels.
Changes in cardiometabolic phenotype
To our knowledge, this is the first study to prospectively evaluate changes in body composition with MRI during trastuzumab. After just 3 months of chemotherapy and trastuzumab therapy, both visceral and intermuscular fat volume were significantly increased. Both fat deposition locations carry independent risk for metabolic dysfunction including insulin resistance, dyslipidaemia, and atherogenesis, as well as cardiovascular morbidity and mortality,25,26 including for women with early-stage breast cancer, irrespective of pre-existing cardiovascular risk factors and cancer treatment.9
Another novel metabolic finding was that we observed very low circulating IGF-1 at the completion of trastuzumab therapy, with 30% of patients within the 2.5th percentile for healthy women27 compared with 11% at baseline. The growth hormone/IGF-1 axis plays a key role in muscle mass regulation,28 and low IGF-1 levels are associated with a substantially higher risk of cardiovascular death, possibly through adverse effects on visceral adiposity and dyslipidemia.29 Taken together, our observed changes to the cardiometabolic phenotype may provide a pathophysiological basis for the increased risk of cardiovascular events in breast cancer survivors and suggest that clinicians should promote healthy lifestyle choices, including access to cardio-oncology rehabilitation during trastuzumab therapy.
Limitations
Our small sample size and low number of LV dysfunction events precluded predictive analysis for cardiotoxicity, yet the high-quality and reproducible MRI data enabled identification of several important and novel cardiac and cardiometabolic changes. T1 mapping data were not available in 14% of assessments due to difficulty with image acquisition and we did not acquire T2 mapping, another MRI measure of myocardial oedema. However, the early myocardial oedema suggested by the elevated post-cycle 4 ECV fraction is supported by corresponding increases in GDF-15, a biomarker of myocardial inflammation. Also, our patient cohort was relatively young, with a lower prevalence of hypertension and diabetes and therefore may represent a relatively lower-risk population. Finally, changes in cardiometabolic phenotype should be also be evaluated in patients with HER2 negative breast cancer.
Conclusions
Our MRI and circulating biomarkers data suggest that trastuzumab therapy is associated with early LV dysfunction and reversible myocardial inflammation and oedema without concurrent myocardial fibrosis or injury. Visceral and inter-muscular fat accumulate while IGF-1 and muscle volume decrease during trastuzumab, all of which have been associated with higher risk of cardiovascular events in population studies. Bisoprolol and perindopril preserve biventricular function but had no discernable effect on myocardial inflammation.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
The trial was supported by the Canadian Institutes of Health Research, the Alberta Cancer Foundation, and the University Hospital Foundation. Perindopril study drug was provided in-kind by Servier. A.A.K. is supported by Postdoctoral Fellowships from Susan G. Komen Foundation (PDF17483149) and the Canadian Institutes of Health Research. J.A.E. and G.Y.O. are supported by Alberta Innovates-Health Solutions, the Canadian Institutes of Health Research, and/or the Heart and Stroke Foundation of Canada. M.J.H. is supported by the Faculty of Nursing Research Chair in aging and quality of life at the University of Alberta.
Conflict of interest: K.C. is currently an employee of Siemens Healthineers but was a graduate student at the time of the study. J.A.E. reports study funding from Novartis, Servier and Pfizer and grants from Merck, Bayer, Trevena and Amgen. G.Y.O. reports study funding from Amgen. All other authors have no conflicts of interest to disclose.
Supplementary Material
Contributor Information
Amy A Kirkham, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7.
Edith Pituskin, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Cross Cancer Institute, 11560 University Avenue, Edmonton, Alberta, Canada, T6G 1Z2.
Richard B Thompson, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7.
John R Mackey, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Cross Cancer Institute, 11560 University Avenue, Edmonton, Alberta, Canada, T6G 1Z2.
Sheri L Koshman, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7.
Davinder Jassal, Cross Cancer Institute, 11560 University Avenue, Edmonton, Alberta, Canada, T6G 1Z2; University of Manitoba, 727 McDermot Avenue, Winnipeg, Manitoba, Canada, R3E 3P5; St. Boniface Hospital, 409 Taché Avenue, Winnipeg, Manitoba, Canada, R2H 2A6.
Marshall Pitz, Cross Cancer Institute, 11560 University Avenue, Edmonton, Alberta, Canada, T6G 1Z2; University of Manitoba, 727 McDermot Avenue, Winnipeg, Manitoba, Canada, R3E 3P5; CancerCare Manitoba, 675 McDermot Avenue, Winnipeg, Manitoba, Canada, R3E 0V9.
Mark J Haykowsky, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7.
Joseph J Pagano, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7.
Kelvin Chow, Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7; Cardiovascular MR R&D, Siemens Medical Solutions USA Inc., 737 N Michigan Avenue, Chicago, Illinois, USA, 60611.
Albert K Tsui, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7.
Justin A Ezekowitz, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7.
Gavin Y Oudit, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7.
D Ian Paterson, University of Alberta, 8440 112 Street NW, Edmonton, Alberta, Canada, T6G 2R7; Mazankowski Alberta Heart Institute, 11220 83 Avenue NW, Edmonton, Alberta, Canada, T6G 2B7.
References
- 1. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D'Amico R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;353:1673–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res 2012;111:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist 2008;13:620–630. [DOI] [PubMed] [Google Scholar]
- 4. McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med 2007;357:94–95. [DOI] [PubMed] [Google Scholar]
- 5. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ky B, Putt M, Sawaya H, French B, Januzzi JLJr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gulati G, Heck SL, Røsjø H, Ree AH, Hoffmann P, Hagve TA, Norseth J, Gravdehaug B, Steine K, Geisler J, Omland T. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (prevention of cardiac dysfunction during adjuvant breast cancer therapy) study. J Am Heart Assoc 2017;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018;71:2281–2290. [DOI] [PubMed] [Google Scholar]
- 9. Cespedes Feliciano EM, Chen WY, Bradshaw PT, Prado CM, Alexeeff S, Albers KB, Castillo AL, Caan BJ. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol 2019;37:2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cespedes Feliciano EM, Chen WY, Lee V, Albers KB, Prado CM, Alexeeff S, Xiao J, Shachar SS, Caan BJ. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol 2020;6:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol 2017;35:870–877. [DOI] [PubMed] [Google Scholar]
- 12. Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S, Oudit G, Chow K, Pagano JJ, Paterson I. Rationale and design of the multidisciplinary approach to novel therapies in cardiology oncology research trial (MANTICORE 101–Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer 2011;11:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1mapping. Magn Reson Med 2014;71:2082–2095. [DOI] [PubMed] [Google Scholar]
- 14. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115–122. [DOI] [PubMed] [Google Scholar]
- 15. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 16. Barthur A, Brezden-Masley C, Connelly KA, Dhir V, Chan KK, Haq R, Kirpalani A, Barfett JJ, Jimenez-Juan L, Karur GR, Deva DP, Yan AT. Longitudinal assessment of right ventricular structure and function by cardiovascular magnetic resonance in breast cancer patients treated with trastuzumab: a prospective observational study. J Cardiovasc Magn Reson 2017;19:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res 2015;2015:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeCoux A, Lindsey ML, Villarreal F, Garcia RA, Schulz R. Myocardial matrix metalloproteinase-2: inside out and upside down. J Mol Cell Cardiol 2014;77:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yousif NG, Al-Amran FG. Novel Toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc Disord 2011;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laird-Fick HS, Tokala H, Kandola S, Kehdi M, Pelosi A, Wang L, Grondahl B. Early morphological changes in cardiac mitochondria after subcutaneous administration of trastuzumab in rabbits: possible prevention with oral selenium supplementation. Cardiovasc Pathol 2020;44:107159. [DOI] [PubMed] [Google Scholar]
- 21. Grover S, Leong DP, Chakrabarty A, Joerg L, Kotasek D, Cheong K, Joshi R, Joseph MX, DePasquale C, Koczwara B, Selvanayagam JB. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol 2013;168:5465–5467. [DOI] [PubMed] [Google Scholar]
- 22. Demissei BG, Hubbard RA, Zhang L, Smith AM, Sheline K, McDonald C, Narayan V, Domchek SM, DeMichele A, Shah P, Clark AS, Fox K, Matro J, Bradbury AR, Knollman H, Getz KD, Armenian SH, Januzzi JL, Tang WHW, Liu P, Ky B. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc 2020;9:878–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Geisberg CA, Abdallah WM, da Silva M, Silverstein C, Smith HM, Abramson V, Mayer I, Means-Powell J, Freehardt D, White B, Lenihan D, Sawyer DB. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J Card Fail 2013;19:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hausman GJ, Basu U, Du M, Fernyhough-Culver M, Dodson MV. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte 2014;3:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neeland IJ, Ross R, Després J-P, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart J-C, Eckel RH; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–725. [DOI] [PubMed] [Google Scholar]
- 27. Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Körner A, Obermayer-Pietsch B, Hübener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab 2014;99:1712–1721. [DOI] [PubMed] [Google Scholar]
- 28. Taekema DG, Ling CHY, Blauw GJ, Meskers CG, Westendorp RG, de Craen AJ, Maier AB. Circulating levels of IGF1 are associated with muscle strength in middle-aged- and oldest-old women. Eur J Endocrinol 2011;164:189–196. [DOI] [PubMed] [Google Scholar]
- 29. Burgers AMG, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, Dekkers OM. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab 2011;96:2912–2920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.