FIGURE 1.
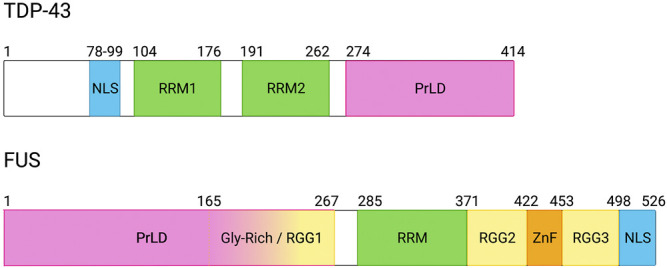
Domain architecture of TDP-43 and FUS. TDP-43 and FUS contain similar structural domains with some notable differences. Both contain a prion-like domain (PrLD), which is characterized by a high concentration of glycine, glutamine, asparagine, and tyrosine amino acids with low sequence complexity; however, the TDP-43 PrLD contains one tyrosine residue whereas the FUS PrLD is comprised of multiple tyrosines that can be phosphorylated. Additionally, the FUS PrLD overlaps with an arginine-glycine-rich (RGG) domain between residues 165 and 267. Notably, FUS contains multiple RGG domains throughout its structure in addition to a zinc-finger motif (ZnF). Both TDP-43 and FUS contain nuclear localization signals (NLS), although they differ in sub-classifications and subsequent nuclear import receptor interactions. Both proteins also contain RNA recognition motifs (RRMs), which are important domains for RNA-binding. Created with BioRender.com.
