Fig. 2. Human biosynthetic pathway for trace amines and catecholamines.
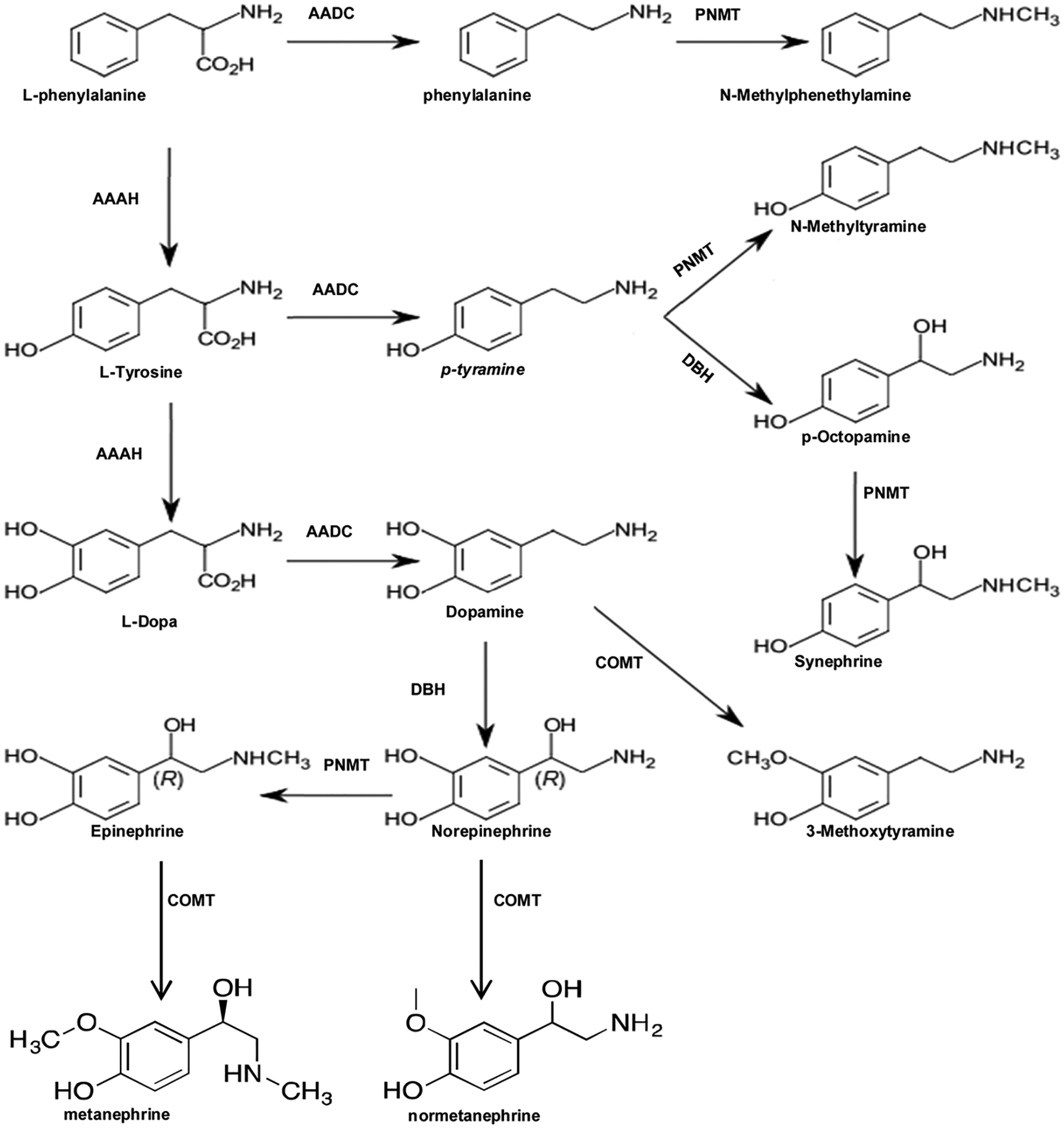
L-Phenylalanine is converted into l-tyrosine by the enzyme AAAH (aromatic amino acid hydroxylases). l-Tyrosine is converted into l-DOPA (l-dihydroxyphenylalanine) by the enzyme AAAH. l-DOPA is catalyzed to dopamine by the enzyme aromatic l-amino acid decarboxylase (AADC). Dopamine itself is also used as a precursor in the synthesis of the neuro-transmitters, norepinephrine and epinephrine. Dopamine is converted into norepinephrine by the enzyme dopamine β-hydroxylase (DBH) and inactivated by COMT Norepinephrine is converted into epinephrine by the enzyme PNMT with SAM as the cofactor. Epinephrine and norepinephrine are inactivated by COMT. [32,39,40].
