Fig. 6.
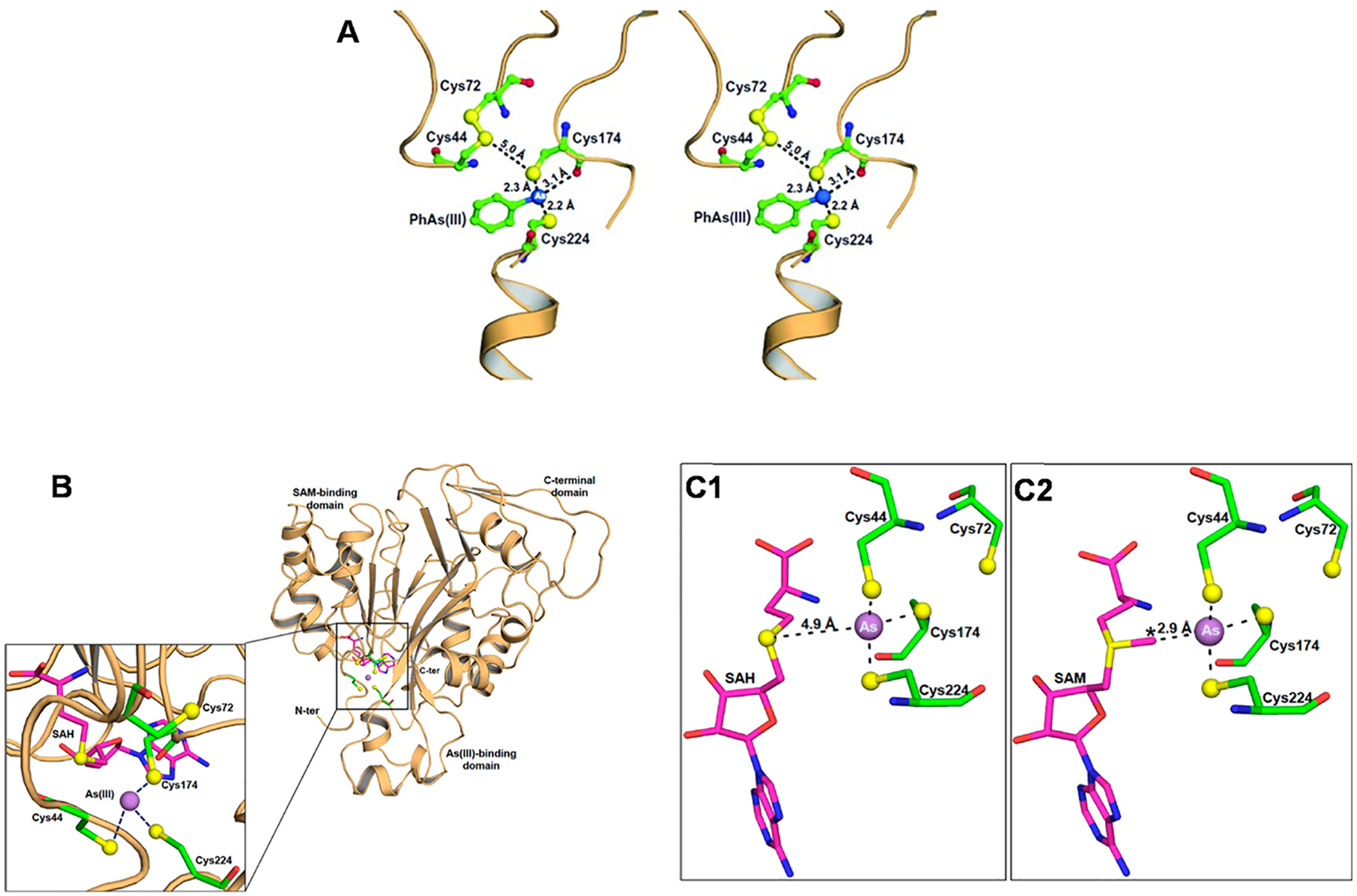
(A) A disulfide bond between Cys44 and Cys72 in the crystal structure of CmArsM with bound aromatic arsenical, phenylarsenite PhAs (III) (PDB entry 4kw7). The conserved cysteine residues are shown in ball-and-stick representation with atoms colored green (carbon), blue (nitrogen) or yellow (sulfur). The dark blue sphere is the As atom. The length of the disulfide bond is approximately 2.1 Å in the PhAs (III)-bound structure. (B) The overall structure of CmArsM consists of an N-terminal domain, an As(III) binding domain, and a C-terminal domain. The inset shows a close-up of the active site showing the four conserved cysteine residues represented by balls and sticks with atoms colored green (carbon), blue (nitrogen), red (oxygen), and yellow (sulfur). The purple sphere is the arsenic atom, and the SAH in the SAM binding site is represented by balls and sticks and with carbon colored magenta. As(III) is bound among conserved residues Cys44, Cys174, and Cys224. (C1) The As atom in the binding site consisting of Cys44, Cys174, and Cys224 is positioned near SAH in the SAM binding site. The distance between the sulfur atom of SAH and the As atom is 4.9 Å. (C2) Distances between the As atom and the sulfur atoms of SAM is 2.9 Å. The S-methyl group is poised for electron transfer from SAM to As(III).
