Figure 1.
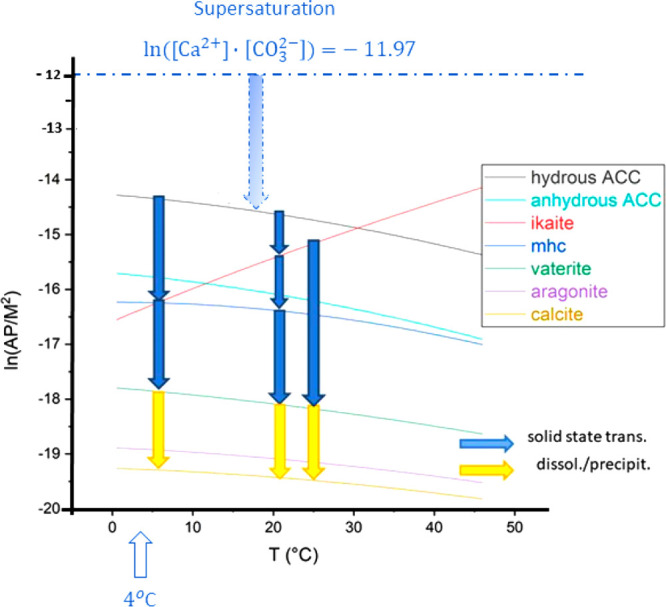
Precipitation diagram of calcium carbonate with possible reaction channels for the formation of metastable calcium carbonate polymorphs under the condition of high supersaturation. The natural logarithm of the activity products of calcium and carbonate ions, ln(AP/M2), is plotted in dependence on the temperature T (°C). The equilibrium curves for the reactions of the various polymorphs have been calculated using equilibrium constants published by Brecevic and Nielsen,19 Bischoff et al.,20 Plummer and Busenberg,21 and Kralj and Brecevic.22 The equilibrium curves of amorphous calcium carbonate (ACC·xH2O) cover a larger range in the diagram depending on the water content x.23,24
