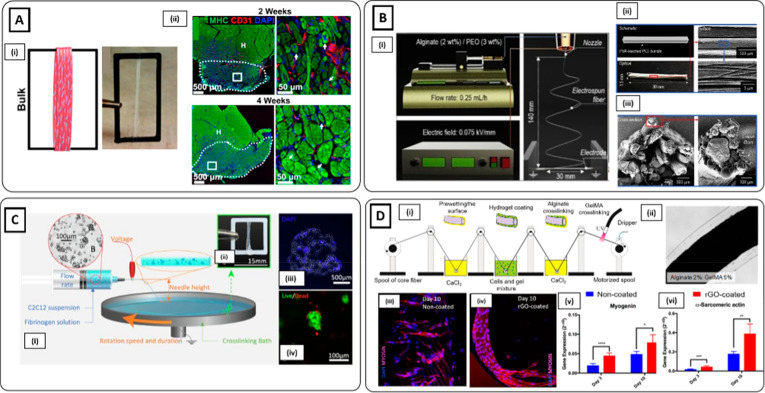
Figure 4.
Hydrogel electrospinning methods. (Ai) C2C12-seeded electrospun fibrinogen bundle collected on ABS frame. (ii) Immunofluorescence staining of MHC (green), CD31 (red), and nuclei (blue) of volumetric muscle loss (VML) defects treated with C2C12-seeded scaffolds at 2 and 4 weeks. High densities of centrally nucleated myofibers and a dense vascularized network were detected (Reproduced with permission from ref (118). Copyright 2018 Elsevier). (Bi) Schematics of alginate/PEO electrospinning process and (ii and iii) schematic, optical, scanning electron microscopy (SEM) images of the muscle-mimetic electrospun bundle structure (Reproduced with permission from ref (120). Copyright 2019 Elsevier). (Ci) Schematic representation of cell electrospinning of C2C12 myoblast agglomerates. (ii) Cell-laden scaffold wrapped around an ABS frame. (iii) Cross-section of cell-laden scaffold on day 0 stained with DAPI (blue). (iv) Live (green) and dead (red) staining of cell-laden bundle showing that cell electrospinning process enabled the preservation of cell viability (Reproduced with permission from ref (123). Copyright 2019 Elsevier). (Di) Schematic of the reel-to-reel fabrication process of cell-laden composite fibers. (ii) Brightfield image of composite microfiber. (iii and iv) MHC immunostaining (red) and (v and vi) transcript levels of myogenic markers assessed higher muscle differentiation in rGO-coated composite microfibers after 10 days of culture (Reproduced with permission from ref (128). Copyright 2019 American Chemical Society).