Scheme 1. Reagents and Conditions:
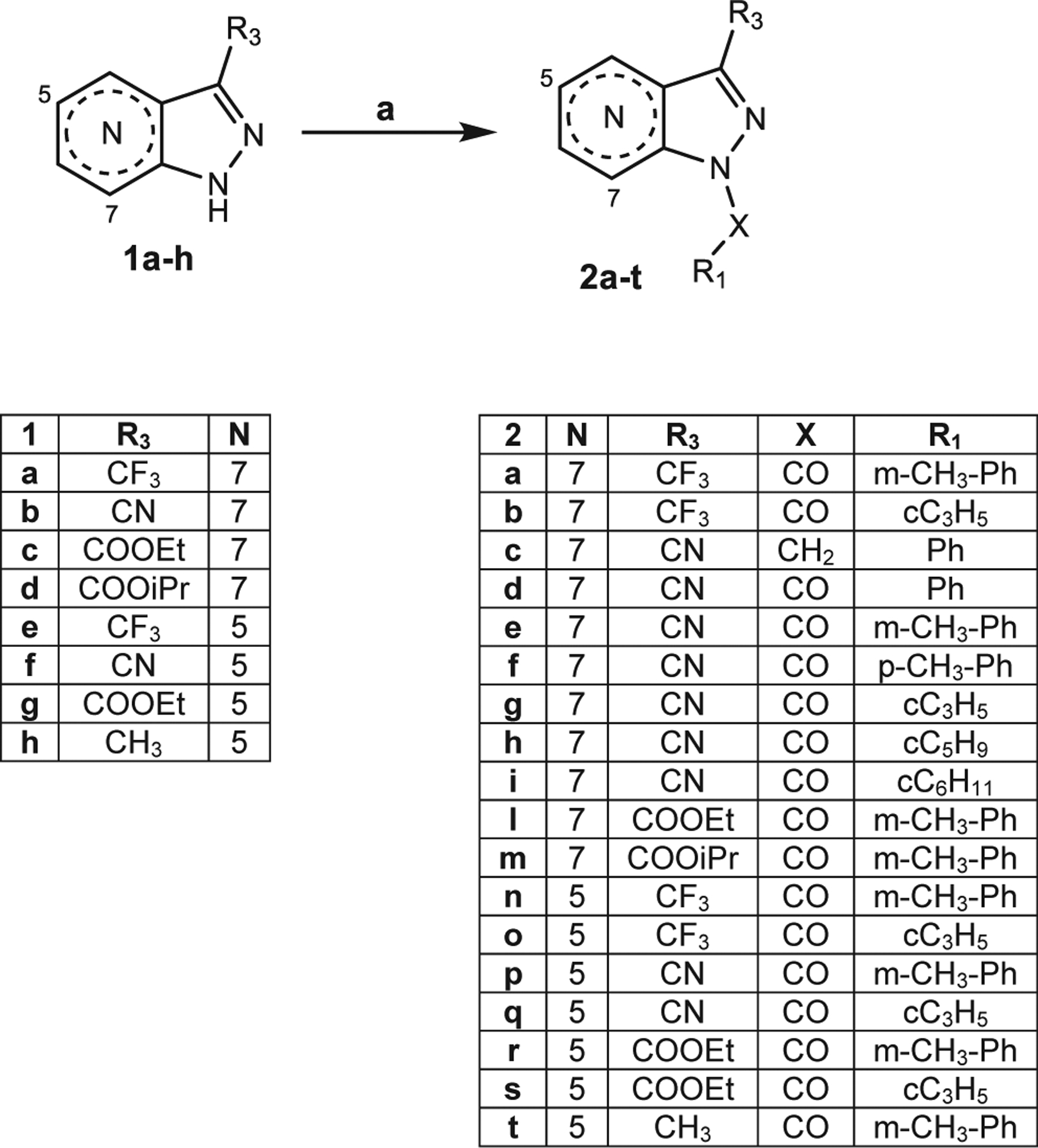
(a) for 2a,b,l-o,r-t: R-COCl, Et3N, anhydrous CH2Cl2, 0 °C, 2 h, then r.t., 2 h; for 2c: Benzyl bromide, anhydrous CH3CN, K2CO3, reflux, 4 h; for 2d: Ph-COOH, dry THF, HOBt, Et3N, DCC, 0 °C, 30′, then r.t., 48 h; for 2e-i,p,q: dry THF, NaH, 0 °C, 30′, then R-COCl, r.t., o/n.
