Table 1.
HNE inhibitory activity of compounds 2a-t, 4, 6, 7, and 9.
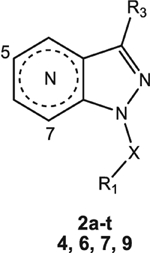
|
|||||
|---|---|---|---|---|---|
| Compound | N | R3 | X | R1 | IC50 (μM)a |
| 2a | 7 | CF3 | CO | m-CH3-Ph | 0.42 ± 0.12 |
| 2b | 7 | CF3 | CO | cC3H5 | 0.050 ± 0.01 |
| 2c | 7 | CN | CH2 | Ph | N.A.b |
| 2d | 7 | CN | CO | Ph | 0.16 ± 0.05 |
| 2e | 7 | CN | CO | m-CH3-Ph | 0.33 ± 0.03 |
| 2f | 7 | CN | CO | p-CH3-Ph | 1.5 ± 0.051 |
| 2g | 7 | CN | CO | cC3H5 | 0.034 ± 0.012 |
| 2h | 7 | CN | CO | cC5H9 | 0.14 ± 0.04 |
| 2i | 7 | CN | CO | cC6H11 | 1.1 ± 0.22 |
| 2l | 7 | COOEt | CO | m-CH3-Ph | 2.0 ± 0.44 |
| 2m | 7 | COOiPr | CO | m-CH3-Ph | 0.98 ± 0.31 |
| 2n | 5 | CF3 | CO | m-CH3-Ph | 0.033 ± 0.011 |
| 2o | 5 | CF3 | CO | cC3H5 | 0.087 ± 0.021 |
| 2p | 5 | CN | CO | m-CH3-Ph | 0.010 ± 0.003 |
| 2q | 5 | CN | CO | cC3H5 | 0.079 ± 0.023 |
| 2r | 5 | COOEt | CO | m-CH3-Ph | 0.016 ± 0.005 |
| 2s | 5 | COOEt | CO | cC3H5 | 0.069 ± 0.026 |
| 2t | 5 | CH3 | CO | m-CH3-Ph | 0.760 ± 0.14 |
| 4 | 7 | NO2 | CO | cC3H5 | 0.021 ± 0.002 |
| 6 | 7 | NH2 | CO | cC3H5 | 9.9 ± 1.3 |
| 7 | 7 | NHCO-cC3H5 | CO | cC3H5 | 1.5 ± 0.14 |
| 9 | 7 | NHCO-m-CH3-Ph | CO | CH3 | 25.2 ± 1.3 |
| Sivelestat | 0.050 ± 0.020 | ||||
| A 20 | – | CN | CO | m-CH3-Ph | 0.007 ± 0.0015 |
IC50 values are presented as the mean ± SD of three independent experiments.
N.A.: no inhibitory activity was found at the highest concentration of compound tested (50 μM).
