Abstract
Background
The duration of viable viral shedding is important to be defined in regards of viral transmission in SARS-CoV-2 infection with the backdrop of the current worldwide effort for revising isolation polices and establishing the duration of infectiousness.
Methods
In this review we searched databases including Medline and google scholar for research articles published between January 2020 and January 2022. We included case reports, case series, cross sectional, cohort, and randomized control trials that reported the duration of shedding of viable SARS-CoV-2 virus. After evaluating the criteria for inclusion, 32 articles (2721 patients) were included.
Result
This review showed that the median for the last day of successful viral isolation was 11 (8.5–14.5 95% CI) , 20 (9.0–57.5 95 %CI), 20 (9.0–103 95 %CI) for the general population, critical patients and immunocompromised individuals, respectively, with significant association between prolonged viral shedding, disease severity (P-Value 0.024) and immunosuppressive status (P-Value 0.023).
The corresponding higher cutoff of CTv to culturable virus ranged between 26.25 and 34.00 (95% confidence interval) with median of 30.5, and higher values were observed when critical (25.0–37.37 95 %CI) and immunocompromised patients (20.0–37.82 95 %CI) have been excluded, this deviation did not represent a statistical significance (P-Value 0.997 and 0.888) respectively.
Conclusion
Our review highlights that repeating SARS-CoV-2 viral RNA test solely in recovering patients has no importance in determining infectivity and emphasizes the individualization of de-isolation decisions based on the host factors and a combined symptom and testing-based approaches with the later benefiting most of correlation with recently introduced rapid antigen test. Our finding in the review also opposes the most recent CDC Guidance on shortening isolation duration in term of the last days of viable transmissible virus, therefore caution should be considered when revising such protocols.
Keywords: COVID-19, PCR, Viral culture, Cycle threshold value, Transmission, Isolation
Abbreviations: RT-PCR, Reverse transcription polymerase chain reaction; RAT, Rapid antigen test; CTv, Cycle thresholdvalue
Introduction
Historically, six strains of coronaviruses are believed to infect humans, of which four strains were identified from upper respiratory tract infection from patients with common cold (Su et al., 2016), the other two, are associated with more severe disease and mortality including severe acute respiratory syndrome (SARS-CoV) and Middle east respiratory syndrome (MERS-CoV) (Cui et al., 2019). The seventh coronavirus was identified as a cause of pneumonia outbreak in Wuhan, China in December 2019 (Zhu et al., 2020), the later three are believed to be zoonotic in origin although the intermediate carrier of severe acute respiratory syndrome coronavirus – 2 (SARS-CoV-2) is not yet precisely known (Surveillances, 2020).
The main route for SARS-CoV-2 transmission is by close contact and respiratory droplet allowing high rate of infectivity, which poses a real challenge on health care systems that necessitates the need of contact tracing and testing. Real time reverse transcription polymerase chain reaction (RT-PCR) has become the standard of SARS-CoV-2 diagnosis because of its high sensitivity and specificity (Wiersinga et al., 2020, Li et al., 2020, Steinbrook, 2020, Shen et al., 2020).
SARS-CoV-2 RNA positivity by RT-PCR has been detected up to 12 weeks, but RT-PCR positivity might not represent the presence of viable virus (Sun et al., 2020). Confirmation of virus replication and infectivity requires viral culture, which is not practical in acute settings as the virus requires prolonged time to be isolated and specific laboratory settings with biosafety levels of 3 or more (Li et al., 2020).
Based on available published literature, the Center of Disease Control and Prevention (CDC) stated that discontinuing isolation can be considered after day 10 of symptoms onset, which can be extended to 20 days for severe cases or immunocompromised individual, awaiting more solid evidence regarding replication-competent virus shedding, which is still an area of debate (CDC. Coronavirus Disease, 2019, CDC. Coronavirus Disease, 2019). Currently, two years into the pandemic in the post vaccination and boosting era when the surge of Omicron variant predominated the active cases worldwide, the CDC shortened the quarantine period to five days CDC, Media Releases, 2021. While this step is rationalized and endorsed by some infectious diseases authorities IDSA, 2022, it is still not fully acceptable by medical communities. (AMA, 2022)
Method
Search strategy and selection criteria
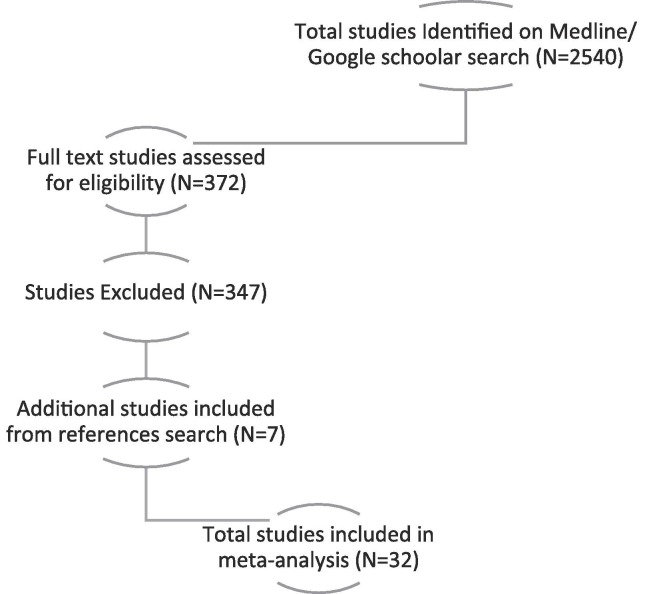
We conducted a comprehensive search on both Medline and google scholar database up to January 2022, using keywords “COVID-19” Or “SARS-CoV-2” Or “Coronavirus” combined with “Culture” or “Virus Isolation” with the use of keywords’ Medical Subject Heading (MESH) And truncation. The total studies screened were 2540, of which 372 full text studies were assessed for eligibility.
The total studies included in the review were 32 after exclusion of 347 articles with seven additional studies included from references search. The main reason for exclusion included if the study method did not include viral culture, if the purpose of viral isolation was for sequencing, genotyping or phenotyping purpose, if the study reported viral culture form non-respiratory body fluid, or if the last day of successful isolation was not clearly mentioned in the study [Fig. 1. ].
Fig. 1.
Prisma Algorithm for database search and article selection.
Data analysis
We reviewed the articles to extract the following information from each: First Author, setting, name of the journal, date of article first available online, study sample size, number of cases with successful viral isolation, last day of positive culture, type of sample, cycle threshold Value (CTv) and inclusion of immunocompromised individuals or critically ill patients (Supplementary Table 1). The data was analyzed using SPSS. Median, grouped median, mean values and 95% confidence interval of the analyzed values were calculated using Anova-T test with bootstrapping to number of samples. The level of significance was established based on linear-by-linear association and 2-sided P-value of 0.05 or less.
Results
The systematic search included 2540 possible relevant articles. After evaluating the criteria for inclusion, 32 articles (2721 patients) were included (Supplementary Table 1). Of the 32 articles included, 7 were done in united states, 7 in France, 3 in Canada, 3 in China, 2 in Japan and 1 in each of the following countries (Australia, Germany, Italy, Netherland, South Korea, Spain, Switzerland, Taiwan, Turkey and United Kingdom), 17 articles (53.13%) were available online in 2020, 13 (40.62%) in 2021 and 2 (6.25) in 2022.
Regarding the type of patients included in the study, immunocompromised were included in 12 articles (37.5%), patients with critical disease were included in 15 articles (46.9%) while the remaining 20 (62.5%) and 17 (53.1%) studies excluded or did not specify the inclusion of immunocompromised individuals and critical cases, respectively.
In regard to the type of samples used when assessing viral culture, 29 studies (90.6%) used nasopharyngeal swab, 11 (34.38%) used oropharyngeal swab, sputum samples were used in 5 (15.6) articles and endotracheal aspirates in 3 (9.38%) articles. 1 (3.13%) study tested samples directly from saliva and a combination of methods were used in 14 (43.75%) of the studies included.
In regards to the last day of virus isolation by culture, most of the data retrieved from systematic review of the above-mentioned articles showed that the last day of successful viral isolation ranged somewhere between 8.5 and 14.50 days (95% confidence interval (CI)) with median of 11 days and mean of 28.75 days.
The duration of viral viability appears to be more prolonged in critical patients with median (9.0–57.5 95 %CI) and grouped median (9.0–53.0 95 %CI) of 20 days and mean of 47.5 days and shorter if those patients were not specified of excluded with median and grouped median of 9 days (8.0–13.0 95 %CI) and mean of 10 days, with estimated 2-sided statistical significance of 0.024
A similar pattern is also observed when immunocompromised individuals were included in the reviewed article with calculated median (9.0–103 95 %CI) and grouped median (9.0–85.98 95 %CI) of 20 days and mean of 54.36 days which noticed to be shorted with median (8.0–13.98 95 %CI) and grouped median (8.2–13.33 95 %CI) of 9 days and mean of 11.67 if this category of patient was not specified or excluded (P-Value 0.023).
When looking at the relation of CTv and positive viral culture, the corresponding higher border of CTv to culturable virus ranged between 26.25 and 34.00 (95% confidence interval) with median of 30.5, grouped median of 30.67 and mean of 30.82, although this range was slightly wider when critical cases (25.0–37.37 95 %CI) and immunocompromised patients (20.0–37.82 95 %CI) have been excluded with median of 32.5, but this deviation did not represent a statistical significance or linear-by-linear association (P-Value 0.997 and 0.888) respectively (Supplementary Table 2).
Discussion
This narrative review provides data on the understanding of SARS-CoV-2 infectivity. The findings show that 95% of samples are no longer viable after day 15 in the general population, with a median of 11 days, and with a mean of 28.75 days. When applying this review on critical patients and immunocompromised individuals, the viable viral shedding could last up to 2 and 4 months, respectively, with a median of 20 days. These findings correlate with the initial suggestions made from the Center of Disease Control, that the duration of infectiousness lasts up to 10 days for mild to moderate cases and up to 20 days in severe cases or severely immunocompromised hosts, and materializes liberal shortening of the period of quarantine into an exclamatory question (CDC, 2016, CDC. Coronavirus Disease, 2019).
To date, the diagnosis of COVID-19 has relied on the detection of SARS-CoV-2 through molecular detection (Wiersinga et al., 2020, Li et al., 2020, Steinbrook, 2020, Shen et al., 2020). While this method is the gold standard by the World Health Organization (WHO) as it is both rapid and highly sensitive (Asrani et al., 2021), there are important limitations in the context of both duration and rate of positivity. Noticeably, this discrepancy has been reflected in some reviewed studies that showed that patients with SARS-CoV-2 continue to have prolonged viral shedding even up to 60 days, no live virus was isolated by culture method beyond 18 days (Million et al., 2020). Similarly, out of 84 positive RT-PCR for SARS-CoV-2 in healthcare workers, only one sample produces a cytopathic effect on the 11th day of incubation (Longtin et al., 2022). The use of RT-PCR as a follow-up method for SARS-CoV-2 infection might represent dead viral particles and has led to infer misleading information regarding the duration of infectivity of patients, defining cases and isolation policies. This subsequently increases the burden on healthcare utilities and human resources, and needs to be further interpreted with caution to minimize its impact (Asrani et al., 2021, Widders et al., 2020).
Vero cells were used primarily for the identification of causative viruses of the disease and evaluating vaccine effectiveness for such viruses. Recently, Vero E6 cell line was found to enhance SARS-CoV-2 In vitro growth by exposing the virus to selective pressure. Although the use of cell culture to define the virus infectivity has been criticized as it may overexpresses the viral growth and not truly represent the viral pathogenesis and transmutability in the human host (Wurtz et al., 2021, Basile et al., 2020, Ramirez et al., 2021), the study of viral growth in human airway epithelium revealed comparable viral dynamics and rebutted this theory (Milewska et al., 2020). Importantly, not all studies using cell culture for SARS-CoV-2 identification reported the viral cytopathic effect, which serves as an important role for delineating viruses’ pathogenicity (Longtin et al., 2022). However, the ability to use viral culture to determine infectivity remains challenging due to unavailable resources and the labor-intensive nature of the pandemic.
A relationship is apparent between the lower CTv threshold and positive SARS-CoV-2 viral culture (La Scola et al., 2020), with higher cutoff of 34 based on most of the published articles (Million et al., 2020, La Scola et al., 2020, Lescure et al., 2020, Gautret et al., 2020). In this review the analysis showed that 95% of the viable samples have the higher cutoff of CTv less than 35 (25.25–34.6) for the total cohort representing the general population with a median of 30.5. these findings are supported by similar reviews that correlated lower CTv to infectivity, although, on the contrary, correlation to disease severity was not established statically in our review (Rao et al., 2020). Therefore, according to available evidence; infection prevention and control guidelines may take into account that CTv equal or above 35 may be safely discharged and no longer require isolation.
As confirmed by multiple reviews, disease severity and immunosuppressive status are widely accepted as independent risks for prolonged viral shedding (van Kampen et al., 2021 Jan 11, Feng et al., 2020). This observation has been confirmed by our review which found that a viable virus can be isolated from this group of patients for up to 2–4 months with a median of 20 days. While the lengthened duration in critical patients is attributed to multiple factors, including the mechanical ventilation dynamics, the nature of samples and the use of immunomodulators (Xu et al., 20202020) in immunosuppressed host, this finding is proposed to be secondary to delayed seroconversion due to underlying immunosuppressive disease or therapy (Buder et al., 2021, Murphy and Dzik, 2020 Nov 12). However, the decision of discontinuing isolation in such an entity of patients remains an ample challenge. Though a combination of test based along with symptoms-based approaches could help guide the decision-making process in such entities of patients as proposed by Alshukairi et al. (Alshukairi et al., 2021 Jul).
The recent introduction of point of care testing including rapid antigen test (RAT), for example the Panbio™ COVID-19 Ag Rapid Test Device (Abbott Diagnostic GmbH, Jena, Germany) started to be widely available as it is self-operated, easily interpreted and highly specific, furthermore, it is more sensitive with lower CTv (less than 25), which is more determinate of infectiousness, as some recent reports suggested that PCR-positive/RAT-negative individuals are less likely to have transmissible diseases, its use in correlation with SARS-CoV-2 RT-PCR might serve as important de-isolation tool, specifically in patient with prolonged molecular test positivity (Strömer et al., 2020 Dec 28, Fenollar et al., 2021 Jan 21, Albert et al., 2021 Mar).
There were several limitations in this systematic review, most of the studies were of low to moderate quality, importantly, the articles’ data were heterogeneous in regards to disease severity and patient population. Also, some studies did not specifically report the relationship between CTv and successful virus isolation by culture, but correlated it rather to RT-PCR. Despite a huge effort done from the start of the pandemic, further studies regarding SARS-CoV-2 transmissibility dynamics are needed, especially in post-vaccination era.
Conclusion
We are providing data on the dynamics of SARS-CoV-2 infectivity. Our review showed that 95% of samples are no longer viable after day 15 in the general population with a median of 11 days. On the other hand, the viable viral shedding could last up to 2 and 3–4 months in critical patients and immunocompromised individuals, respectively, with a median of 20 days, The prolonged viral shedding observed in our study opposes the most recent CDC Guidance on shortening isolation duration. Therefore, caution should be considered when revising such protocols.
This review also clearly shows that repeat testing SARS-CoV-2 viral RNA in recovering patients has no importance in determining infectivity. This emphasizes the use of a multi-tier approach for isolation discontinuation which incorporates patient host factors, disease symptomatology and severity. Thus, benefiting most from a combination of molecular and serological testing to guide clinical decisions and infection control policies rather than the sole use of molecular testing.
Funding information
This study was not financially funded by any governmental, medical or pharmaceutical agencies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinpr.2022.100140.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M.Á., et al. Field evaluation of a rapid antigen test (PanbioTM covid-19 ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2021;27(3):472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshukairi A.N., Tolah A.M., Dada A., Al-Tawfiq J.A., Almagharbi R.S., Saeedi M.F., et al. Test-based de-isolation in COVID-19 immunocompromised patients: Cycle threshold value versus SARS-CoV-2 viral culture. Int. J. Infectious Diseases. 2021;108:112–115. doi: 10.1016/j.ijid.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMA: CDC quarantine and isolation guidance is confusing, counterproductive [Internet]. American Medical Association. [cited 2022 Jan 8]. Available from: https://www.ama-assn.org/press-center/press-releases/ama-cdc-quarantine-and-isolation-guidance-confusing-counterproductive.
- Asrani P., Eapen M.S., Chia C., Haug G., Weber H.C., Hassan M.I., Sohal S.S. Diagnostic approaches in COVID-19: clinical updates. Expert Rev. Respiratory Med. 2021;15(2):197–212. doi: 10.1080/17476348.2021.1823833. [DOI] [PubMed] [Google Scholar]
- Basile K, McPhie K, Carter I, Alderson S, Rahman H, Donovan L, et al. Cell-based culture of SARS-CoV-2 informs infectivity and safe de-isolation assessments during COVID-19. Clin Infect Dis. 2020 Oct 24;ciaa1579. [DOI] [PMC free article] [PubMed]
- Buder F, Bauswein M, Magnus CL, Audebert F, Lang H, Kundel C, et al. SARS-CoV-2 infectivity correlates with high viral loads and detection of viral antigen and is terminated by seroconversion. J Infect Dis. 2021 Aug 24;jiab415.
- Cdc newsroom [Internet]. CDC. 2016 [cited 2022 Jan 8]. Available from:.
- CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Retrieved from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
- CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Retrieved from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html.
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Li J., Yao S., Yu Q., Zhou W., Mao X., Li H., Kang W., Ouyang X., Mei J.i., Zeng Q., Liu J., Ma X., Rong P., Wang W. Clinical factors associated with progression and prolonged viral shedding in covid-19 patients: a multicenter study. Aging Dis. 2020;11(5):1069. doi: 10.14336/AD.2020.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., et al. Evaluation of the panbio covid-19 rapid antigen detection test device for the screening of patients with covid-19. J. Clin. Microbiol. 2021;59(2):e02589–e2620. doi: 10.1128/JCM.02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P., Hocquart M., Eldin C., Finance J., Vieira V.E., Tissot-Dupont H.T., Honoré S., Stein A., Million M., Colson P., La Scola B., Veit V., Jacquier A., Deharo J.-C., Drancourt M., Fournier P.E., Rolain J.-M., Brouqui P., Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B., Le Bideau M., Andreani J., Hoang V.T., Grimaldier C., Colson P., Gautret P., Raoult D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V., Houhou-Fidouh N., Valette M., Mailles A., Lucet J.-C., Mentre F., Duval X., Descamps D., Malvy D., Timsit J.-F., Lina B., van-der-Werf S., Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhao C., Bao J., Tang B., Wang Y., Gu B. Laboratory diagnosis of coronavirus disease-2019 (COVID-19) Clin. Chim. Acta. 2020;510:35–46. doi: 10.1016/j.cca.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtin Y., Charest H., Quach C., Savard P., Baz M., Boivin G., Farfard J., Villeneuve J., Roger M., De Serres G. Infectivity of healthcare workers diagnosed with coronavirus disease 2019 (COVID-19) approximately 2 weeks after onset of symptoms: A cross-sectional study. Infect. Control Hosp. Epidemiol. 2022;43(1):102–104. doi: 10.1017/ice.2020.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska A., Kula-Pacurar A., Wadas J., Suder A., Szczepanski A., Dabrowska A., Owczarek K., Marcello A., Ochman M., Stacel T., Rajfur Z., Sanak M., Labaj P., Branicki W., Pyrc K., Sandri-Goldin R.M. Replication of severe acute respiratory syndrome coronavirus 2 in human respiratory epithelium. J. Virol. 2020;94(15) doi: 10.1128/JVI.00957-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honoré S., Stein A., Jacquier A., Deharo J.-C., Chabrière E., Levasseur A., Fenollar F., Rolain J.-M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille. France. Travel Medicine and Infectious Disease. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.F., Dzik S. COVID-19, plasma, and hypogammaglobulinemia. Blood. 2020;136(20):2245–2246. doi: 10.1182/blood.2020008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S., Fernandez-Antunez C., Galli A., Underwood A., Pham L.V., Ryberg L.A., Feng S., Pedersen M.S., Mikkelsen L.S., Belouzard S., Dubuisson J., Sølund C., Weis N., Gottwein J.M., Fahnøe U., Bukh J. Overcoming Culture Restriction for SARS-CoV-2 in Human Cells Facilitates the Screening of Compounds Inhibiting Viral Replication. Antimicrob. Agents Chemother. 2021;65(7) doi: 10.1128/AAC.00097-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.N., Manissero D., Steele V.R., Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of covid-19. Infect Dis Ther. 2020;9(3):573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Zhou Y., Ye J., Abdullah AL-maskri A.A., Kang Y., Zeng S.u., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10(2):97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrook R. Contact tracing, testing, and control of covid-19—learning from taiwan. JAMA Intern Med. 2020;180(9):1163. doi: 10.1001/jamainternmed.2020.2072. [DOI] [PubMed] [Google Scholar]
- Strömer A., Rose R., Schäfer M., Schön F., Vollersen A., Lorentz T., et al. Performance of a point-of-care test for the rapid detection of sars-cov-2 antigen. Microorganisms. 2020;9(1):58. doi: 10.3390/microorganisms9010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xiao J., Sun R., Tang X., Liang C., Lin H., Zeng L., Hu J., Yuan R., Zhou P., Peng J., Xiong Q., Cui F., Liu Z., Lu J., Tian J., Ma W., Ke C. Prolonged persistence of sars-cov-2 rna in body fluids. Emerg. Infect. Dis. 2020;26(8):1834–1838. doi: 10.3201/eid2608.201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- “Transmission & Isolation.” Accessed February 16, 2022. https://www.idsociety.org/covid-19-real-time-learning-network/infection-prevention/isolation-and-infection-control/.
- van Kampen J.J.A., van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat. Commun. 2021;12(1):267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widders A., Broom A., Broom J. SARS-CoV-2: The viral shedding vs infectivity dilemma. Infection, Disease & Health. 2020;25(3):210–215. doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wurtz N., Penant G., Jardot P., Duclos N., La Scola B. Culture of SARS-CoV-2 in a panel of laboratory cell lines, permissivity, and differences in growth profile. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(3):477–484. doi: 10.1007/s10096-020-04106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W., Li Y., Ni Q., Zou R., Li X., Xu M., Zhang Y., Zhao H., Zhang X., Yu L., Su J., Lang G., Liu J., Wu X., Guo Y., Tao J., Shi D., Yu L., Cao Q., Ruan B., Liu L., Wang Z., Xu Y., Liu Y., Sheng J., Li L. Factors associated with prolonged viral rna shedding in patients with coronavirus disease 2019(COVID-19) Clin. Infect. Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.