Keywords: blood-brain barrier, brain edema, cerebral blood flow, ischemia, matrix metalloproteinase-9, neurobehavioral outcomes, tight junction, transcranial ultrasound
Abstract
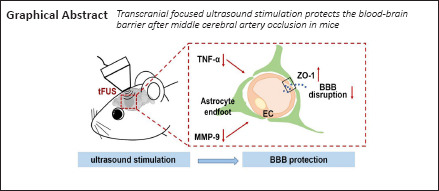
Blood-brain barrier (BBB) disruption underlies the vasogenic edema and neuronal cell death induced by acute ischemic stroke. Reducing this disruption has therapeutic potential. Transcranial focused ultrasound stimulation has shown neuromodulatory and neuroprotective effects in various brain diseases including ischemic stroke. Ultrasound stimulation can reduce inflammation and promote angiogenesis and neural circuit remodeling. However, its effect on the BBB in the acute phase of ischemic stroke is unknown. In this study of mice subjected to middle cerebral artery occlusion for 90 minutes, low-intensity low-frequency (0.5 MHz) transcranial focused ultrasound stimulation was applied 2, 4, and 8 hours after occlusion. Ultrasound stimulation reduced edema volume, improved neurobehavioral outcomes, improved BBB integrity (enhanced tight junction protein ZO-1 expression and reduced IgG leakage), and reduced secretion of the inflammatory factors tumor necrosis factor-α and activation of matrix metalloproteinase-9 in the ischemic brain. Our results show that low-intensity ultrasound stimulation attenuated BBB disruption and edema formation, which suggests it may have therapeutic use in ischemic brain disease as a protector of BBB integrity.
Introduction
Stroke, including ischemic and hemorrhagic types, is the second leading cause of death worldwide and has a high risk of disability, of which ischemic stroke accounts for approximately 76% (Campbell et al., 2019; Zhong et al., 2020; Virani et al., 2021). Tissue plasminogen activator is the only FDA-approved medication for ischemic stroke but is limited by a short therapeutic window and has a high risk of hemorrhage (Miller et al., 2011; Liu et al., 2016). Other therapeutic approaches are necessary, including ones that can attenuate ischemic brain injury.
One of the pathological phenomena that occurs during ischemic stroke is blood-brain barrier (BBB) dysfunction (Li et al., 2019; Alamri et al., 2021). The BBB consists of endothelial cells, pericytes, astrocytic end-foot processes, the basement membrane, and tight junctions (Pan et al., 2020). It separates the central nervous system (CNS) from the blood circulation, maintaining a microenvironment appropriate for neurons and glial functioning. When ischemic stroke occurs, BBB disruption occurs and cytotoxic edema develops, which leads to infiltration of hematogenous fluid from the blood circulation into the brain via disrupted tight junctions (vasogenic edema), as well as regional neuroinflammation (Obermeier et al., 2013; Zhang et al., 2020). To date, several potential therapeutic approaches have been developed to attenuate BBB disruption (Abdulkadir et al., 2020). In animal studies, researchers have discovered drugs that reduce hyperpermeability by inhibiting inflammatory mediators, decreasing matrix metalloproteinases (MMPs), and increasing angiopoetin-1 to enhance tight junctions (Jiang et al., 2018; Zhang et al., 2020). MMP-9 is a gelatinase found in blood and microvessel wells that can cause degradation of tight junction proteins during ischemic brain injury. Inhibiting hyperexpression of MMP-9 might attenuate BBB disruption (Qi et al., 2016). However, such a treatment would be difficult in clinical practice because of the narrow therapeutic time window and safety issues.
Increasing evidence has shown that physical treatments may also facilitate BBB repair after ischemic brain injury. Such treatments include transcranial magnetic stimulation, transcranial direct current stimulation, optogenetics, and focused ultrasound stimulation (FUS) (Klomjai et al., 2015; Bergmann et al., 2016; Jiang et al., 2017; Wang et al., 2021). Among these, FUS has obvious advantages as a non-invasive method with superior spatial specificity and deeper penetration. Early application mainly focused on high-intensity FUS in surgery; however, over the past decade, use of low-intensity FUS for neuromodulation has increased (Bystritsky and Korb, 2015). Compared to high-intensity, low-intensity FUS has lower power and minimal thermal effects (ter Haar, 2007; Jiang et al., 2019). Low-intensity FUS has been demonstrated to activate neuronal circuits in hippocampus in vitro and ex vivo without tissue damage (Tyler et al., 2008; Jiang et al., 2019). Furthermore, low-intensity FUS can stimulate intact brain circuits without surgery in mice (Tufail et al., 2010). Low-intensity FUS has also been applied in CNS disease models. After ischemic stroke in rats, its application results in a significant neuroprotective effect (Schuhmann et al., 2018). However, its effect on the BBB after ischemic brain injury remains unknown.
We hypothesized that transcranial FUS (tFUS) attenuates brain edema in a mouse model of transient middle cerebral artery occlusion (tMCAO). To investigate this, we utilized a unique tFUS technique to treat tMCAO mice and explore the effect of tFUS on: 1) neurological deficits and cerebral edema in the acute phase of occlusion; 2) BBB injury and tight junction proteins; and 3) activity of MMP-9.
Materials and Methods
Experimental design
Experimental animal studies were reported according to the Animal Research: Reporting of in vivo Experiments guidelines (Percie du Sert et al., 2020). Animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University, Shanghai, China (approval No. BME-ll-2019052) on May 20, 2019. Animals were housed in a specific pathogen free level animal facility at 26 ± 1°C in a 12-hour dark/light cycle. The individual ventilated cages contained four to five mice per cage. Adult male C57BL/6J mice weighing 25 to 30 g were used in the study experiments (n = 76, purchased from Charles River Laboratories, China [license No. SCXK (Jing) 2019-0009]). tMCAO was performed for 90 minutes to induce a focal ischemic brain injury. To assess the general condition of mice, body weight was measured using an electronic scale before surgery and 1 and 3 days after surgery. The mice in the experimental groups underwent 10 minutes of tFUS 2, 4, and 8 hours after occlusion, respectively. Neurobehavioral outcomes were evaluated in a double-blinded fashion using the modified neurological severity score (mNSS) on days 1 and 3 after tMCAO. Brain infarct volume and edema formation were determined using cresyl violet staining. BBB permeability was measured using IgG leakage and tight junction protein expression. MMP-9 activity was detected using a gelatin zymography assay.
tFUS in a mouse model of tMCAO
Seventy-six mice were divided into the following 6 groups (12–13 mice per group) using the random number table method: sham, sham/tFUS, tMCAO alone, tMCAO/tFUS at 2 hours, tMCAO/tFUS at 4 hours, and tMCAO/tFUS at 8 hours. Mice were anesthetized with 1.5% isoflurane (RWD Life Science, Shenzhen, China) in air under spontaneous breathing conditions. After the mice were fixed on the stereotaxic apparatus, tFUS was applied to the left hemisphere (ipsilateral to the side of occlusion) for 10 minutes with the probe attached to the overlying skull (Figure 1A). The pulsed wave bursts were delivered from a single-element focused ultrasound transducer (diameter, 2.5 cm; geometrical focal length, 12.44 mm) (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, China). Ultrasound was induced using the following parameters: fundamental frequency, 500 kHz; spatial peak temporal average intensity, 39 mW/cm2; pulse width, 500 μs; pulse interval,1 ms; stimulation duration, 300 ms; and stimulation interval, 3 seconds (Figure 1B and C).
Figure 1.
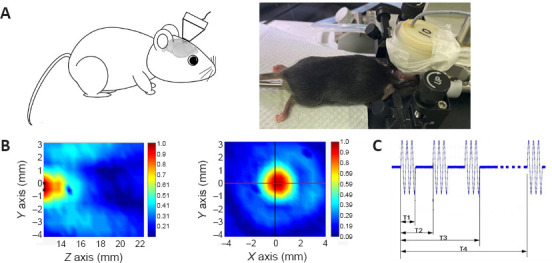
The low-frequency tFUS setup on the mouse ischemic brain.
(A) The illustration and photograph show a tFUS transducer placed on the skull overlying the cerebral hemisphere affected by transient occlusion (left). (B) Distribution field of ultrasound intensity. The Z axis represents the depth of stimulation. The X and Y axes represent the width of stimulation. The colors represent normalized ultrasound intensity: red is high and blue is low. (C) Schematic diagram showing the tFUS sequence. T1 (pulse width) = 500 μs. T2 (pulse interval) = 1 ms. T3 (stimulation duration) = 300 ms. T4 (stimulation interval) = 3 seconds. tFUS: Transcranial focused ultrasound stimulation.
tMCAO procedure
The detailed tMCAO procedure has been described previously with minor modification (Lu et al., 2017). Animals were anesthetized with 1.5% isoflurane in air under spontaneous breathing conditions. Body temperature was maintained at 37.0 ± 0.5°C during surgery using a heating pad (RWD Life Science). The entire surgical procedure was performed under a surgical microscope (Leica, Wetzlar, Germany). A midline incision was made in the neck to expose and separate the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA). A round head 6-0 suture (Dermalon, 1741-11, Covidien, OH) coated with silicone was gently inserted into the ICA from the ECA stump to induce MCA occlusion. The suture insertion distance from the bifurcation to the left MCA opening was 9.5 ± 0.5 mm. Successful occlusion was defined as a 20% reduction in cerebral blood flow (CBF) as determined by laser speckle imaging (LSI, Figure 2A). After 90 minutes of occlusion, the suture was withdrawn, the CCA restored, and blood flow returned to at least 70% of baseline blood. Sham surgery mice underwent the same procedure without suture insertion.
Figure 2.
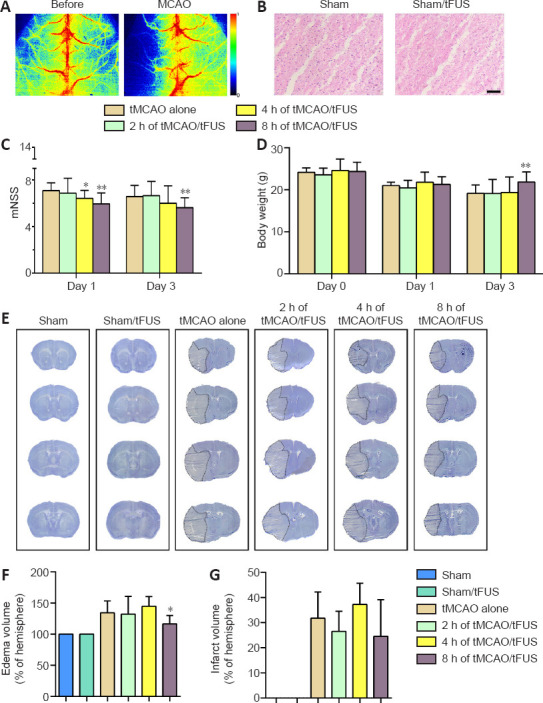
tFUS reduces brain edema and neurological deficit.
(A) Laser speckle imaging of mouse brain tissue before and after tMCAO. The colors from blue to red represent normalized blood flow velocity from low to high. (B) Hematoxylin and eosin staining of brain coronal sections in sham and tFUS-treated mice. Scale bar: 50 μm. The area shown, which was 2 mm beneath the surface of the cortex, received the focus of tFUS. Cellular morphology appears normal without cell death or inflammation. Bar graphs show modified neurological severity score (C, n = 7–12) and body weight (D, n = 7–12) in each group. (E) Cresyl violet stained brain sections on day 3 in the six study groups. The dotted area indicates the area of infarction. Bar graphs show edema volume (F, n = 5–8) and infarct volume (G, n = 5–8). All experiments were repeated three times. Data are expressed as means ± standard deviation. *P < 0.05, **P < 0.01, vs. tMCAO alone (one-way analysis of variance followed by Bonferroni correction). All experiments were repeated three times. tFUS: Transcranial focused ultrasound stimulation; tMCAO: transient middle cerebral artery occlusion.
LSI
To confirm the success of tMCAO, we monitored surface blood flow using LSI. The detailed procedure has been described previously with minor modification (Wang et al., 2019). Mice were fixed on the stereotaxic apparatus and anesthetized with 1.5% isoflurane in air under spontaneous breathing conditions. After scalp shaving and incising the skin to expose the skull, a camera and laser beam (785 nm) of the LSI system (RWD Life Science) were placed straight above the mouse brain. Imaging of CBF was obtained before tMCAO, during tMCAO, 10 minutes before tFUS treatment, and 10 minutes after tFUS treatment.
Western blot analysis
To explore tight junction function, we determined expression of zonula occludens-1 (ZO-1), occludin, and claudin-5 using Western blot analysis. The ipsilateral cortex was collected into a protein lysate to extract protein. The protein (30 μg) was loaded onto a 10% Acryl/Bis-acryl (Shanghai Epizyme Biotechmology Co., Ltd., Shanghai, China) gel for electrophoresis. Proteins were transferred to PVDF membranes (Merck Milliopore, Tullagreen, Ireland) activated by methanol. Membranes were incubated with 5% non-fat milk for 1 hour at room temperature followed by overnight incubation with primary antibodies to ZO-1 (Cat# 61-7300, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific, Waltham, MA, USA), occludin (Cat# 33-1500, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific), claudin-5 (Cat# 35-2500, anti-mouse, 1:1000 dilution, Thermo Fisher Scientific), and β-actin (Cat# 66009, anti-mouse, 1:2000 dilution, Proteintech, Wuhan, China) at 4°C on the shaker. Membranes were washed with tris-buffered saline with 0.1% Tween 20 (pH 7.5) 3 times and then incubated with horseradish peroxidase-conjugated secondary antibodies (Cat# HA1001, donkey anti-rabbit, 1:5000 dilution; Cat# HA1006, donkey anti-mouse, 1:5000 dilution, HuaAn Biotechnology Co., Ltd., Hangzhou, China) at room temperature for 1 hour on the shaker. The membranes were reacted with an enhanced chemiluminescence substrate (Meilunbio, Dalian, China) and then imaged (Criterion Stain Free System Instruction Manual, https://www.bio-rad.com/webroot/web/pdf/lsr/literature/10014472.pdf, Bio-Rad, Hercules, CA, USA).
Gelatin zymography assay
To determine MMP activity, we measured MMP-2 and MMP-9 activity using a gelatin zymography assay. The detailed assay procedure has been described previously with minor modification (Cai et al., 2017). An equal amount of protein from the perifocal ischemic region (60 μg) was loaded onto 10% Acryl/Bis-acryl gel with 15% gelatin (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for electrophoresis. The sodium dodecyl sulfate was removed from the gels with 2.5% Triton X-100 for 1 hour. Gels were incubated in 1× incubation buffer (10×: 0.5 M Tris Base, 2.0 M NaCl, 0.05 M CaCl2, 0.2% (w/v) Brij-35, pH 7.6, Sinopharm Chemical Reagent Co., Ltd.) for 72 hours at 37 °C. Then, they were stained with Coomassie stain (Sinopharm Chemical Reagent Co., Ltd.) for 1 hour followed by destaining in 10% acetic acid for 2 hours. Results were recorded using a scanner (Bio-Rad, Hercules, CA, USA).
Infarct and edema volume measurement
Brain infarct volume was measured by cresyl violet (Meilunbio) staining as described in a previous study (Mamtilahun et al., 2020). The mouse brains were rapidly removed and frozen at –80°C. A series of coronal sections were cut (20 μm thickness) using a cryostat (Leica Biosystems, Shanghai, China) and collected. Sixteen sections were calculated in total. The following formula was used to calculate infarct volume:
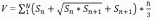
where h is the distance (μm) between two adjacent sections (200 μm) and S is the infarct area (mm2) in each section as determined by ImageJ software (64-bit Java 1.8.0_172, NIH, Bethesda, MD, USA; Schneider et al., 2012). Edema volume was determined using the same method. Both infarct and edema volume are expressed as a percentage of the total volume of the ipsilateral hemisphere.
Hematoxylin and eosin staining to evaluate brain injury
Hematoxylin and eosin (HE) staining was performed to examine the histopathology following brain tissue injury. Frozen coronal sections were fixed with 4% paraformaldehyde for 10 minutes, stained using a HE kit (#MB9898, Meilunbio), and examined using bright-field microscopy (Leica).
Immunostaining
To further explore tight junction function, ZO-1, occludin, and claudin-5 expression were also examined using immunostaining. Frozen coronal sections were fixed with ice methanol at –20°C for 10 minutes followed by washing with phosphate buffered saline three times. Sections were incubated with 10% bovine serum albumin for 1 hour at room temperature and then incubated overnight with primary antibodies to CD31 (Cat# AF3628, anti-mouse, 1:200 dilution, R&D Systems, Minneapolis, MN), ZO-1 (Cat# 61-7300, anti-mouse, 1:100 dilution, Thermo Fisher Scientific), occludin (Cat# 33-1500, anti-mouse, 1:100 dilution, Thermo Fisher Scientific), and claudin-5 (Cat# 35-2500, anti-mouse, 1:100 dilution, Thermo Fisher Scientific) at 4°C. The primary antibodies were washed with phosphate buffered saline 3 times and then incubated with donkey anti-mouse IgG H&L (Alexa Fluor® 594, Cat# A21203, 1:500, Thermo Fisher Scientific) and donkey anti-mouse IgG H&L (Alexa Fluor® 488, Cat# A21202, 1:500, Thermo Fisher Scientific) conjugated secondary antibodies at 37°C for 1 hour. The results were observed under a confocal microscope (Leica).
IgG staining
To determine the effect of ultrasound on BBB integrity, we examined IgG leakage. Frozen coronal sections were fixed with 4% paraformaldehyde for 10 minutes and 0.3% H2O2 in methanol for 30 minutes. The ABC reagent kit (Vector Labs, Burlingame, CA, USA) and DAB kit (Vector Labs) were used in the following steps. Results were examined using bright-field microscopy (Leica) and analyzed using ImageJ software to calculate mean integrated optical density (IOD) (Tang et al., 2014).
Real-time polymerase chain reaction assay
To demonstrate the inflammatory response in the brain, we examined interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β). The ipsilateral cortex was collected and placed into TRIzol reagent (Thermo Fisher Scientific) to extract RNA. The RNA concentration was detected using a spectrophotometer (NanoDrop1000, Thermo Fisher, Wilmington, DE). RT of RNA to cDNA was performed using a RT kit (Yeasen, Shanghai, China). The cDNA was used to perform real-time PCR with the One Step RT-qPCR SYBR Green Kit (Yeasen). The RT-PCR amplification reaction was performed under the following conditions: 95°C for 5 minutes followed by 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds. The primer sequences (Sangon Biotech, Shanghai, China) are listed in Table 1 (Wen et al., 2020). The primer sequence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was designed by Sangon Biotech (No. 662304) and used as an internal control and the expression level of each target gene was normalized to that of GAPDH using the 2–ΔΔCt method, where Ct is the threshold cycle.
Table 1.
Primer sequences of IL-1β, TNF-α, and TGF-β used in this study
| Gene | Primer (5’–3’) |
|---|---|
| IL-1β | Forward: TAC ATC AGC ACC TCA CAA GC |
| Reverse: AGA AAC AGT CCA GCC CAT ACT | |
| TNF-α | Forward: ACC CTC ACA CTC AGA TCA TCT T |
| Reverse: GGT TGT CTT TGA GAT CCA TGC | |
| TGFβ | Forward: CAC CGG AGA GCC CTG GAT A |
| Reverse: TGT ACA GCT GCC GCA CAC A |
IL-1β: Interleukin-1β; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α.
Neurobehavioral assessment
mNSS scores (Schaar et al., 2010; Tang et al., 2014) ranging from 0 to 14 were used to assess neurological deficit. The tests were performed on days 1 and 3 after tMCAO and included raising the mouse by the tail (0–3 points), walking on the floor (0–3 points), beam balance testing (0–6 points), and reflex testing (0–2 points). Higher scores represent worse neurobehavioral function.
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0 (IBM, Armonk, NY, USA). All data are presented as means ± standard deviation. The open source G*Power version 3.1.9.7 was used to estimate the minimal sample size and perform power analysis (Faul et al., 2007). Data were compared using the Student's t-test or one-way analysis of variance followed by Bonferroni correction. P < 0.05 was considered significant.
Results
tFUS improves neurobehavioral outcomes and reduces edema in tMCAO mice
We first examined HE-stained brain tissue to assess the effect of tFUS and found that tFUS-treated mouse brain had no detectable pathological changes, the same as normal control mouse brain (Figure 2B). To explore the effect of tFUS on mouse neurobehavioral outcomes, mice were treated with tFUS for 10 minutes 2, 4, and 8 hours after tMCAO. We found that mNSS on days 1 and 3 after tMCAO did not differ between mice treated with tFUS 2 hours after tMCAO (tMCAO/tFUS at 2-hour group) and tMCAO alone mice. The tMCAO/tFUS at 4-hour group performed significantly better than the tMCAO alone group on day 1 (P < 0.05) but not on day 3. mNSS score was significantly lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group on days 1 and 3 (P < 0.01; Figure 2C). These results demonstrated that tFUS attenuated neurological deficit in tMCAO mice compared to control mice.
We also examined animal body weight before surgery and on days 1 and 3 following tMCAO. Before tMCAO and 1 day after, body weight did not differ among the tMCAO alone and the three tMCAO/tFUS groups. However, on day 3 after tMCAO, body weight was significantly lower in the tMCAO alone group than the tMCAO/tFUS at 8-hour group (P < 0.05; Figure 2D), suggesting that tFUS can improve mouse general condition after ischemic brain injury.
To determine if better neurological outcome in the tMCAO/tFUS at 8-hour group was associated with less brain tissue injury, we examined brain infarct volume and edema formation (Figure 2E). Edema volume was smaller in the tMCAO/tFUS at 8-hour group than the tMCAO alone group on day 3 after tMCAO (P < 0.05; Figure 2F). However, edema volume did not differ among the tMCAO/tFUS at 2 hours, tMCAO/tFUS at 4 hours, and tMCAO alone groups. Infarct volume did not differ among groups (P > 0.05; Figure 2G).
tFUS improves CBF
To investigate the effect of tFUS on CBF, we measured CBF in the normal mouse brain using LSI 10 minutes before and 10 minutes after tFUS stimulation. tFUS caused an approximately 20% increase in CBF (Figure 3A), indicating a direct effect on CBF. We then measured CBF in tMCAO mice before and after tFUS. CBF consistently changed in tMCAO mice. Compared to the tMCAO alone group, CBF increased in the tMCAO/tFUS at 8 hours group (Figure 3B), indicating that tFUS stimulation applied 8 hours after occlusion improved CBF.
Figure 3.
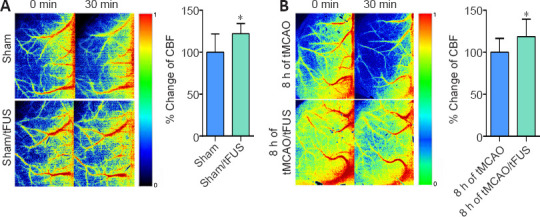
tFUS improves CBF.
(A) Laser speckle imaging (LSI) shows CBF in sham and sham/tFUS mice. Bar graph shows semi-quantified data of fractional changes in blood flow. n = 8 per group. (B) CBF in the tMCAO group and tMCAO/tFUS at 8 hours group. Bar graph shows semi-quantified data. 0 min means immediately after tFUs and 30 min means 30 minutes after tFUS in tFUS groups. In the control groups, they mean the corresponding time with the tFUS groups. Data are expressed as means ± standard deviation. n = 7–9 per group. *P < 0.05, vs. tMCAO (Student's t-test). All experiments were repeated three times. CBF: cerebral blood flow; tFUS: Transcranial focused ultrasound stimulation; tMCAO: transient middle cerebral artery occlusion.
tFUS attenuates BBB disruption in tMCAO mice
To determine whether tFUS treatment reduced tMCAO-induced brain edema by protecting the BBB, we examined BBB leakage using IgG staining. We did not detect IgG leakage in the tFUS-treated normal mice, indicating that tFUS itself did not affect BBB permeability. However, degree of IgG leakage was smaller in the tMCAO/tFUS at 8-hour group compared to the tMCAO alone group on day 3 after tMCAO (P < 0.05; Figure 4A). This suggests that tFUS treatment reduced brain edema by attenuating disruption of the BBB in tMCAO mice.
Figure 4.
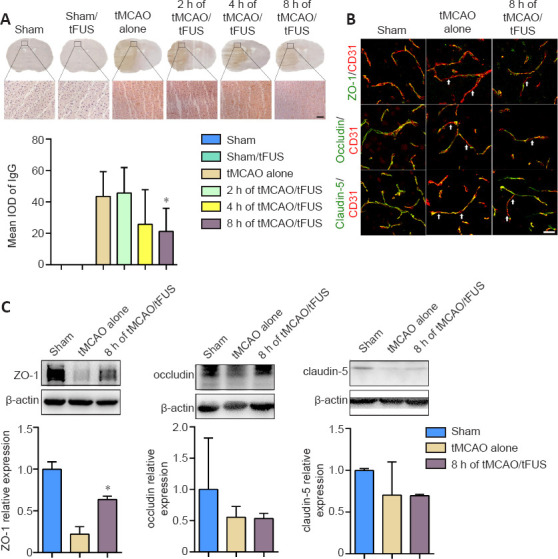
tFUS treatment reduces IgG leakage and enhances ZO-1 expression in tMCAO mice.
(A) IgG staining of brain coronal sections 3 days after tMCAO in the tMCAO alone, tMCAO/tFUS at 2 hours, tMCAO/tFUS at 4 hours, tMCAO/tFUS at 8 hours, and control groups. Brown color indicates IgG leakage from the cerebral vasculature. The bottom row images show IgG immunostaining in the respective boxes of the upper row, which represents the perifocal ischemic region. Scale bar: 50 μm. Bar graph shows the semi-quantified mean integrated optical density data. n = 5–7 per group (one-way analysis of variance followed by Bonferroni correction). (B) CD31/ZO-1, CD31/occludin, and CD31/claudin-5 double immunostaining in the perifocal ischemic region in the sham, tMCAO alone, and tMCAO/tFUS at 8 hours (8 h of tMCAO/FUS) groups. White arrows indicate discontinuous labeling and gap formation. Scale bar: 25 μm. (C) Western blot bands show expression of the tight junction proteins ZO-1, occludin, and claudin-5 in brain tissue collected from the perifocal ischemic region on day 3 after tMCAO. Molecular weights: ZO-1: 220 kDa, occludin: 52 kDa, claudin-5: 23 kDa, and β-actin: 42 kDa. Bar graphs show the semi-quantified Western blot data. All experiments were repeated three times. Data are expressed as means ± standard deviation. n = 3 per group. *P < 0.05, vs. tMCAO alone (one-way analysis of variance followed by Bonferroni correction). All experiments were repeated three times. tFUS: Transcranial focused ultrasound stimulation; tMCAO: transient middle cerebral artery occlusion.
We then further examined whether tFUS treatment protected the structural integrity of the BBB. Expression of the tight junction proteins ZO-1, occludin, and claudin-5 was examined in tMCAO mice using immunostaining and Western blotting 3 days after occlusion. Expression of ZO-1, but not occludin or claudin-5, was significantly higher in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P < 0.05; Figure 4B and C). These results suggest that tFUS attenuated BBB disruption by reducing tight junction protein ZO-1 degradation.
tFUS decreases MMP-9 activity and TNF-α expression
To demonstrate whether the protective effects of tFUS on the BBB in ischemic mice were associated with decreased MMP-9 activity, we examined MMP-9 enzyme activity in tMCAO mice 3 days after occlusion using the gelatin zymography assay. MMP-9 activity was lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P < 0.05, Figure 5A), suggesting that the decrease in tight junction degradation induced by tFUS may be related to reduction in MMP-9 activity.
Figure 5.
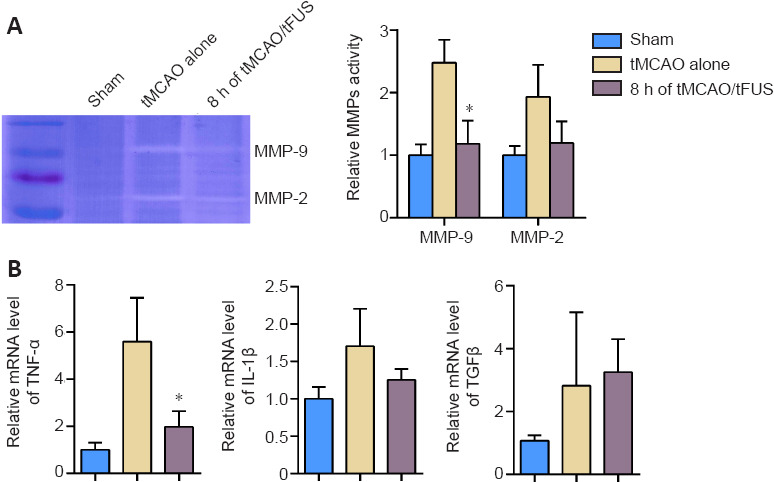
MMP-9 activity and TNF-α expression decrease in ischemic brain after tFUS treatment.
(A) Zymography shows MMP-9 and MMP-2 activity in the mouse brain tissue from the sham, tMCAO alone, and tMCAO/tFUS at 8-hour groups on day 3 following occlusion. Bar graph shows the semi-quantified MMP-9 and MMP-2 activity. n = 3 per group. (B) Bar graphs show the relative mRNA expression of TNF-α, IL-1β, and TGF-β normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) measured on day 3 following occlusion by real-time quantitative PCR. Data are expressed as means ± standard deviation. n = 3–4 per group. *P < 0.05, vs. tMCAO alone (one-way analysis of variance followed by Bonferroni correction). All experiments were repeated three times. IL-1β: Interleukin-1β; MMP: matrix metalloproteinases; tFUS: Transcranial focused ultrasound stimulation; TGF-β: transforming growth factor-β; tMCAO: transient middle cerebral artery occlusion; TNF-α: tumor necrosis factor-α.
We also examined mRNA expression of the cytokines IL-1β, TNF-α, and TGF-β on day 3 after occlusion and found that expression of TNF-α, but not IL-1β or TGF-β, was significantly lower in the tMCAO/tFUS at 8-hour group than the tMCAO alone group (P < 0.05; Figure 5B). This suggests that tFUS can attenuate focal inflammation.
Discussion
In this study, we demonstrated that tFUS can modulate the sensorimotor circle to promote improved neurobehavioral outcome in a mouse model of tMCAO. tFUS reduced vasogenic edema volume, which may result from improved cortical blood flow. tFUS also reduced BBB leakage and attenuated tight junction protein ZO-1 loss, which may be related to reduction of MMP-9 activity and TNF-α expression.
tFUS is a relatively new technique and determining the best stimulation parameters is important to maximize beneficial effects while limiting side effects. Ultrasound can be divided into low (20 kHz–1 MHz) and high frequencies (1–20 MHz) (Zhao et al., 2019). Low-frequency ultrasound waves have deeper penetration (Chen et al., 2015). Focused ultrasound is used in various tissue therapies and has shown promise in transcranial neuromodulation. Unfocused ultrasound is commonly used for imaging and diagnostic purposes (Xin et al., 2016; Zhao et al., 2019). tFUS frequencies generally range from 0.2 to 1.5 MHz (Mehic et al., 2014). For the best neuromodulatory effect in brain disease treatment, tFUS intensity should range from 30 to 500 mW/cm2 (Tengfei et al., 2015; Oh et al., 2019; Liu et al., 2020). After several tests, we finally selected a frequency and intensity of 0.5 MHz and 39 mW/cm2, respectively, for our study. We did not observe any visible behavioral abnormalities after tFUS in normal mice, nor did we detect morphological changes in the brain tissue. At this frequency and intensity, tFUS exhibited high spatial resolution, deep penetration, and no thermal brain injury, indicating appropriateness for small animal application.
tFUS applied immediately after occlusion can reduce edema volume in a rat model of distal MCAO (Guo et al., 2015). In addition, tFUS treatment protects BBB integrity in a mouse model of traumatic brain injury (Su et al., 2017). Furthermore, earlier tFUS intervention after ischemic stroke in rats provides a stronger neuroprotective effect (Liu et al., 2019, 2020). The duration of ultrasound application in the above studies ranged from 5 to 60 minutes; therefore, after our preliminary experiment, we selected a duration of 10 minutes and applied it 2, 4, and 8 hours after tMCAO.
Our results demonstrated that tFUS intervention 8 hours after tMCAO resulted in the best reduction of brain injury; the earlier time points (2 and 4 hours after tMCAO) were not as effective. Brain edema occurs rapidly after ischemic stroke and includes cytotoxic and vasogenic types. Cytotoxic edema is caused by a water distribution shift from extracellular to intracellular compartments. Vasogenic edema occurs because of continuous BBB injury in the first few hours after stroke. Our results showed that tFUS at 2 and 4 hours after tMCAO had no better effect than tFUS at 8 hours, which suggests that tFUS interrupts vasogenic edema rather than cytotoxic edema. Therefore, tFUS may have a relatively long therapeutic window for treating ischemic stroke and other diseases that induce vasogenic edema. However, the mechanisms underlying the effects of tFUS on brain edema warrant further investigation.
We demonstrated that vasogenic edema decreased in the mouse brain following application of tFUS 8 hours after tMCAO. Moreover, this decrease was associated with a decrease in degradation of the tight junction protein ZO-1. Tight junctions play an important role in maintaining BBB integrity. Notably, degradation of occludin or claudin-5 was not decreased after tFUS treatment, suggesting that the effect of tFUS on the BBB is relatively minor.
Neuroinflammation interacts with activation of MMPs (Rempe et al., 2016). We found that MMP-9 activity and TNF-α expression were decreased in tMCAO mice who received tFUS 8 hours after occlusion, which is consistent with degradation of tight junction proteins. Tight junction degradation could be inhibited via decreased MMP-9 activation. We also found that CBF increased in tFUS treated mice compared to control mice; however, there was no evidence of a link between MMP-9 expression and CBF change. A previous study reported that tFUS can upregulate endothelial nitric oxide synthase (eNOS) in mouse models of bilateral common carotid artery stenosis and vascular dementia (Eguchi et al., 2018). NO concentration can be regulated by eNOS at a physical level. Low NO concentration can activate MMP-9 while a high concentration can inhibit it (O’Sullivan et al., 2014). tFUS may increase eNOS, then increase CBF, and finally inhibit MMP-9 activation. Although the results demonstrated that tFUS is a unique tool for the treatment of ischemic stroke, the optimal therapeutic time, duration, stimulation perimeters need to be further determined. In addition, molecular mechanisms of tFUS induction, such as eNOS, MMPs, growth factors, as well as signal pathways, how these factors involved in neuroprotection and neuronal repair also need be studied.
In conclusion, tFUS can decrease BBB disruption in mice with ischemic stroke. When applied 8 hours after tMCAO, tFUS reduced vasogenic edema, loss of tight junction proteins, MMP-9 activity, and expression of TNF-α. tFUS has therapeutic potential in ischemic stroke.
Acknowledgments:
Li-Yuan Ren and Hang Song (both from Shanghai Jiao Tong University) are acknowledged for their kindly help with ultrasound parameter measurement.
Footnotes
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81802232 (to JXW), 81801170 (to YHT), 82071284 (to YHT), 2019YFA0112000 (to YHT), the Scientific Research and Innovation Program of Shanghai Education Commission, No. 2019-01-07-00-02-E00064 (to GYY), Scientific and Technological Innovation Act Program of Shanghai Science and Technology Commission, No. 20JC1411900 (to GYY), Science and Technology Commission of Shanghai, No. 19441907900 (to JFS).
References
- 1.Abdulkadir RR, Alwjwaj M, Othman OA, Rakkar K, Bayraktutan U. Outgrowth endothelial cells form a functional cerebral barrier and restore its integrity after damage. Neural Regen Res. 2020;15:1071–1078. doi: 10.4103/1673-5374.269029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamri FF, Al Shoyaib A, Syeara N, Paul A, Jayaraman S, Karamyan ST, Arumugam TV, Karamyan VT. Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regen Res. 2021;16:1244–1251. doi: 10.4103/1673-5374.301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann TO, Karabanov A, Hartwigsen G, Thielscher A, Siebner HR. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: Current approaches and future perspectives. Neuroimage. 2016;140:4–19. doi: 10.1016/j.neuroimage.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Bystritsky A, Korb AS. A review of low-intensity transcranial focused ultrasound for clinical applications. Curr Behav Neurosci Rep. 2015;2:60–66. [Google Scholar]
- 5.Cai H, Ma Y, Jiang L, Mu Z, Jiang Z, Chen X, Wang Y, Yang GY, Zhang Z. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther. 2017;25:1448–1459. doi: 10.1016/j.ymthe.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Li CH, Wang YX, Zhang CH, Dong Z, Zhang FF, Wang JH, Zhang PL. Safety and effectiveness of intravenous thrombolysis with recombinant tissue plasminogen activator in 80 years and older acute ischemic stroke patients. Cell Biochem Biophys. 2015;72:883–888. doi: 10.1007/s12013-015-0556-1. [DOI] [PubMed] [Google Scholar]
- 8.Eguchi K, Shindo T, Ito K, Ogata T, Kurosawa R, Kagaya Y, Monma Y, Ichijo S, Kasukabe S, Miyata S, Yoshikawa T, Yanai K, Taki H, Kanai H, Osumi N, Shimokawa H. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia -crucial roles of endothelial nitric oxide synthase. Brain Stimul. 2018;11:959–973. doi: 10.1016/j.brs.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Li H, Lv Y, Lu H, Niu J, Sun J, Yang GY, Ren C, Tong S. Pulsed transcranial ultrasound stimulation immediately after the ischemic brain injury is neuroprotective. IEEE Trans Biomed Eng. 2015;62:2352–2357. doi: 10.1109/TBME.2015.2427339. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Li W, Mamtilahun M, Song Y, Ma Y, Qu M, Lu Y, He X, Zheng J, Fu Z, Zhang Z, Yang GY, Wang Y. Optogenetic inhibition of striatal gabaergic neuronal activity improves outcomes after ischemic brain injury. Stroke. 2017;48:3375–3383. doi: 10.1161/STROKEAHA.117.019017. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, Chen J. A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng. 2019;66:2704–2718. doi: 10.1109/TBME.2018.2889669. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann Phys Rehabil Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Lin S, Chen X, Huang W, Li Q, Zhang H, Chen X, Yang S, Jin K, Shao B. The prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis. 2019;10:544–556. doi: 10.14336/AD.2018.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res. 2016;7:209–219. doi: 10.1007/s12975-016-0459-5. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Du J, Zheng T, Hu S, Dong Y, Du D, Wu S, Wang X, Shi Q. Protective effect of low-intensity transcranial ultrasound stimulation after differing delay following an acute ischemic stroke. Brain Res Bull. 2019;146:22–27. doi: 10.1016/j.brainresbull.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Du J, Zheng T, Hu S, Zhao M, Wang X, Wu S, Wu S, Shi Q. Readout-segmented echo-planar diffusion-weighted MR at 3. 0T for the evaluation the effect of low-intensity transcranial ultrasound on stroke in a rat model. Magn Reson Imaging. 2020;67:79–84. doi: 10.1016/j.mri.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Jiang L, Li W, Qu M, Song Y, He X, Zhang Z, Yang GY, Wang Y. Optogenetic inhibition of striatal neuronal activity improves the survival of transplanted neural stem cells and neurological outcomes after ischemic stroke in mice. Stem Cells Int 2017. 2017 doi: 10.1155/2017/4364302. 4364302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamtilahun M, Wei Z, Qin C, Wang Y, Tang Y, Shen FX, Tian HL, Zhang Z, Yang GY. DL-3n-butylphthalide improves blood-brain barrier integrity in rat after middle cerebral artery occlusion. Front Cell Neurosci. 2020;14:610714. doi: 10.3389/fncel.2020.610714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehic E, Xu JM, Caler CJ, Coulson NK, Moritz CT, Mourad PD. Increased anatomical specificity of neuromodulation via modulated focused ultrasound. PLoS One. 2014;9:e86939. doi: 10.1371/journal.pone.0086939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller DJ, Simpson JR, Silver B. Safety of thrombolysis in acute ischemic stroke: a review of complications, risk factors, and newer technologies. Neurohospitalist. 2011;1:138–147. doi: 10.1177/1941875211408731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Sullivan S, Medina C, Ledwidge M, Radomski MW, Gilmer JF. Nitric oxide-matrix metaloproteinase-9 interactions: biological and pharmacological significance--NO and MMP-9 interactions. Biochim Biophys Acta. 2014;1843:603–617. doi: 10.1016/j.bbamcr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, Hong GS, Koh W, Kwon J, Hwang ES, Woo DH, Youn I, Cho IJ, Bae YC, Lee S, Shim JW, Park JH, Lee CJ. Ultrasonic neuromodulation via astrocytic TRPA1. Curr Biol. 2019;29:3386–3401. doi: 10.1016/j.cub.2019.08.021. e3388. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Qu M, Li Y, Wang L, Zhang L, Wang Y, Tang Y, Tian HL, Zhang Z, Yang GY. MicroRNA-126-3p/-5p overexpression attenuates blood-brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke. 2020;51:619–627. doi: 10.1161/STROKEAHA.119.027531. [DOI] [PubMed] [Google Scholar]
- 27.Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuhmann MK, Kraft P, Bieber M, Haarmann A, Homola GA, Pham M, Nieswandt B, Stoll G. Influence of thrombolysis on the safety and efficacy of blocking platelet adhesion or secretory activity in acute ischemic stroke in mice. Transl Stroke Res. 2018;9:493–498. doi: 10.1007/s12975-017-0606-7. [DOI] [PubMed] [Google Scholar]
- 31.Su WS, Wu CH, Chen SF, Yang FY. Low-intensity pulsed ultrasound improves behavioral and histological outcomes after experimental traumatic brain injury. Sci Rep. 2017;7:15524. doi: 10.1038/s41598-017-15916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang G, Liu Y, Zhang Z, Lu Y, Wang Y, Huang J, Li Y, Chen X, Gu X, Wang Y, Yang GY. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32:3150–3162. doi: 10.1002/stem.1808. [DOI] [PubMed] [Google Scholar]
- 33.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SI, Tyler WJ. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Lin X, Mu Z, Shen F, Zhang L, Xie Q, Tang Y, Wang Y, Zhang Z, Yang GY. Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics. 2019;9:4923–4934. doi: 10.7150/thno.32676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Li G, Deng L, Mamtilahun M, Jiang L, Qiu W, Zheng H, Sun J, Xie Q, Yang GY. Transcranial focused ultrasound stimulation improves neurorehabilitation after middle cerebral artery occlusion in mice. Aging Dis. 2021;12:50–60. doi: 10.14336/AD.2020.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen RX, Shen H, Huang SX, Wang LP, Li ZW, Peng P, Mamtilahun M, Tang YH, Shen FX, Tian HL, Yang GY, Zhang ZJ. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther. 2020;26:416–429. doi: 10.1111/cns.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin Z, Lin G, Lei H, Lue TF, Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5:255–266. doi: 10.21037/tau.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Zhu L, An C, Wang R, Yang L, Yu W, Li P, Gao Y. The blood brain barrier in cerebral ischemic injury – Disruption and repair. Brain Hemorrhages. 2020;1:34–53. [Google Scholar]
- 42.Zhao GJ, Wang ZR, Lin FZ, Cui YS, Xu SL. The safety and efficacy of tPA intravenous thrombolysis for treating acute ischemic stroke patients with a history of cerebral hemorrhage. Braz J Med Biol Res. 2019;52:e7739. doi: 10.1590/1414-431X20187739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong W, Yuan Y, Gu X, Kim SI, Chin R, Loye M, Dix TA, Wei L, Yu SP. Neuropsychological deficits chronically developed after focal ischemic stroke and beneficial effects of pharmacological hypothermia in the mouse. Aging Dis. 2020;11:1–16. doi: 10.14336/AD.2019.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]


