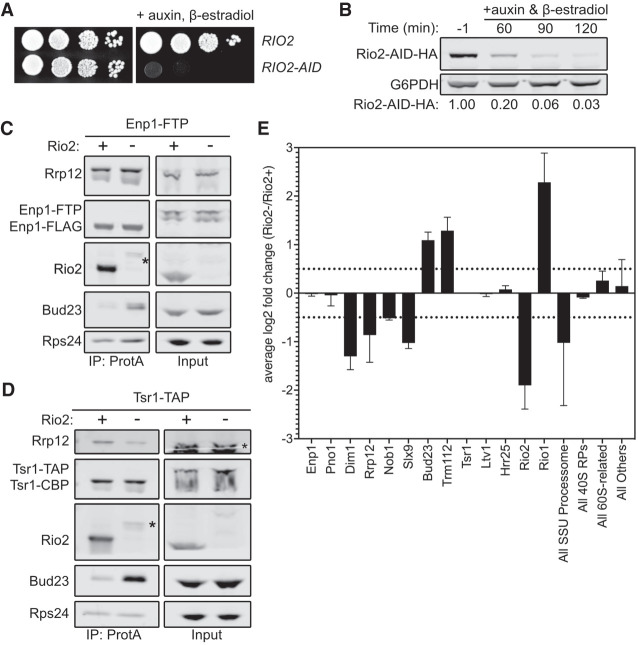
FIGURE 5.
Rio2 is needed for Bud23 release from pre-40S particles. (A) The growth phenotypes of the wild-type (AJY4724) or RIO2-AID (AJY4733) strains as shown by 10-fold serial dilutions of cells on YPD media with or without 0.5 mM auxin and 1 µM β-estradiol. (B) Western blot of time-course of the depletion of Rio2-AID-HA using equivalent amounts of total protein from AJY4733 cells cultured to exponential phase, then collected prior to or after the addition of 0.5 mM auxin and 1 µM β-estradiol for the indicated time points. G6PDH was used as the loading control. The ratio Rio2-AID-HA signal to G6PDH signal for each time point relative to that of the untreated time point (−1) is shown below. (C,D) Bud23 accumulated in Rio2-depleted particles as shown by western blotting for specific factors on pre-40S particles that copurify with Enp1–FTP (C) and Tsr1–TAP (D). Asterisks (*) denote residual Rio2-AID-HA signal that is detected by the anti-Rio2 antibody. For C, strains AJY4724 (Rio2+) and AJY4733 (Rio2−) were cultured in YPD to early exponential phase, then treated with 0.5 mM auxin and 1 µM β-estradiol to deplete Rio2-AID-HA. For D, strains AJY4754 (Rio2+) and AJY4757 (Rio2−) were similarly cultured in YPD. (E) Proteomic composition of Tsr1-associated preribosomal particles as measured by semiquantitative mass spectrometry is shown as the average log2 fold change (Rio2−/Rio2+ samples) for two independent biological replicates. Error bars denote the standard deviation. Individual factors are ordered by the timing of their approximate known association with 40S precursors. The log2 fold change values were calculated as described in the Materials and Methods section. The supporting data are provided in Supplemental File 2.