Fig. 2 |. Predicted effects of thioester charge on phospholipid synthesis.
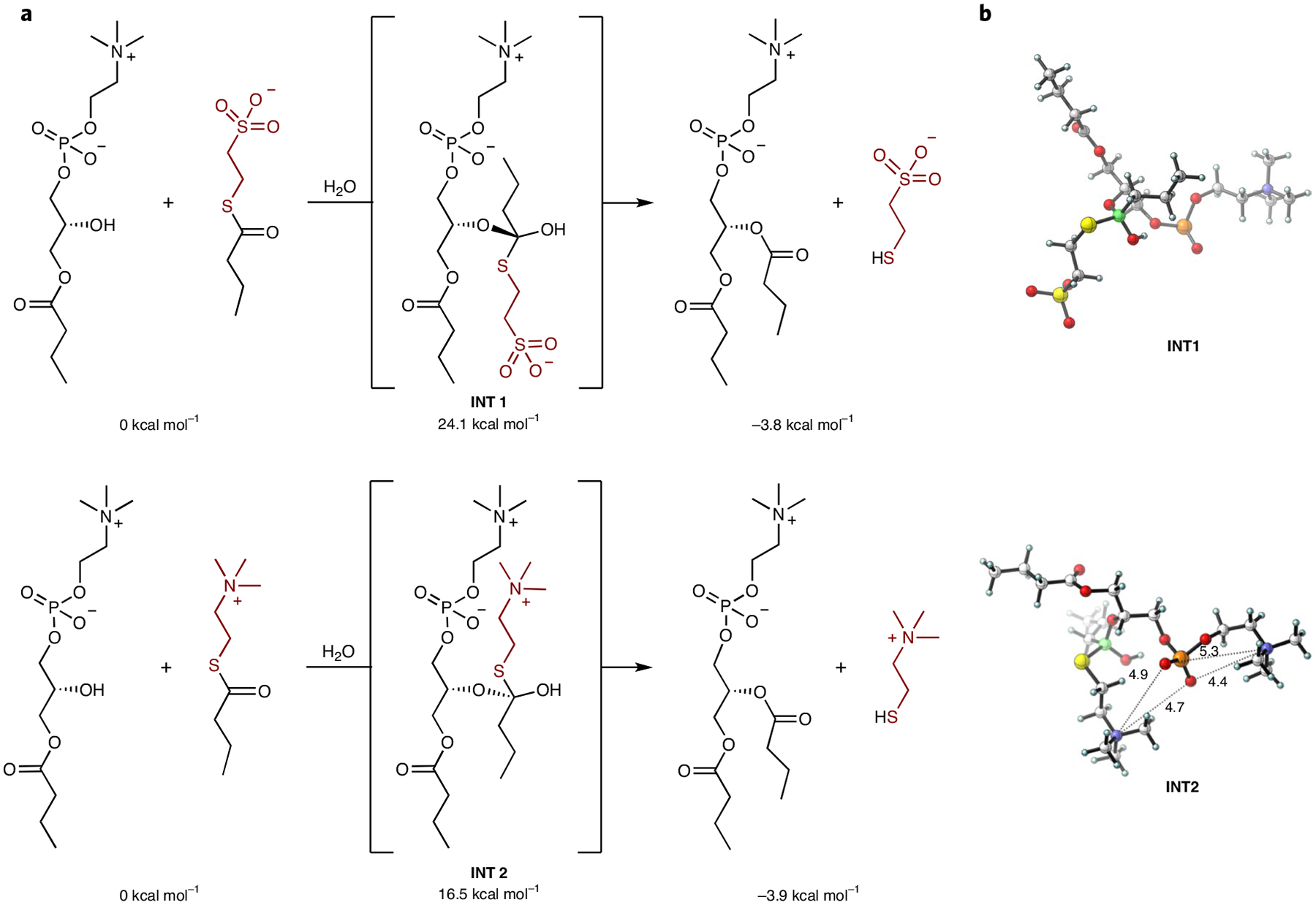
a, Energetics from B3LYP-D3 density functional theory calculations on transacylation reactions. Tetrahedral intermediates iNt1 and iNt2 resemble the transition states of the corresponding addition steps. iNt1 is destabilized by over 7 kcal mol−1 compared with iNt2. b, Optimized structures of reaction intermediates. The negatively charged sulfonate group of iNt1 is distal from the phosphate group to avoid a disfavoured charge repulsion interaction, whereas the positively charged side chain of iNt2 moves into a geometry that will increase the favorable interaction with the phosphate group to stabilize the tetrahedral intermediate. Distances are shown in ångströms.
