ABSTRACT
Ongoing health care-associated outbreaks of the multidrug-resistant yeast Candida auris have prompted the development of several rapid DNA-based molecular diagnostic tests. These tests do not distinguish between live and dead C. auris cells, limiting their use for environmental surveillance and containment efforts. We addressed this critical gap by developing a reverse transcription (RT)-quantitative real-time PCR (RT-qPCR) assay to rapidly detect live C. auris in health care environments. This assay targeted the internal transcribed spacer 2 (ITS2) ribosomal gene by obtaining pure RNA followed by reverse transcription (ITS2 cDNA) and qPCR. ITS2 cDNA was not detectable in bleach-killed cells but was detectable in heat- and ethanol-killed C. auris cells. The assay was highly sensitive, with a detection limit of 10 CFU per RT-qPCR. Validation studies yielded positive cycle threshold (CT) values from sponge matrix samples spiked with 102 to 105 CFU of live C. auris, while dead (bleach-killed) C. auris (105/mL) or other live Candida species (105/mL) had no CT values. Finally, 33 environmental samples positive for C. auris DNA but negative by culture were all negative by RT-qPCR assay, confirming the concordance between culture and the PCR assay. The RT-qPCR assay appears highly reproducible, robust, and specific for detecting live C. auris from environmental samples. The Candida auris RT-qPCR assay could be an invaluable tool in surveillance efforts to control the spread of live C. auris in health care environments.
KEYWORDS: Candida auris, validation, quantitative PCR, reverse transcription
INTRODUCTION
Candida auris is an emerging pathogenic yeast of significant clinical concern because of its frequent intrinsic resistance to fluconazole and other antifungal drugs and the high mortality associated with systemic infections (1–4). The first evidence for C. auris infections in the United States dates to 2013. The New York metropolitan area had an unprecedented outbreak with 50 to 60% mortality in patients with invasive infection (3, 5, 6). Candida auris South Asia clade I was linked to the New York outbreak (7), which is particularly concerning, as some strains of South Asia clade I are now resistant to all three antibiotic classes used to treat fungal infection (8–10). Candida auris has frequently been recovered from environmental surfaces in the rooms of colonized or infected patients (5), and an outbreak of C. auris in an intensive care unit was linked to shared temperature probes (4). The ability of C. auris strains to survive for prolonged periods on dry and moist surfaces has been documented (11). Recent studies showed heavy colonization of the skin and mucous membranes of hospitalized patients and various health care objects by C. auris, suggesting that both patients and contaminated surfaces might be a source of C. auris transmission in the health care setting (6, 12). In 2019, the Centers for Disease Control and Prevention (CDC) added C. auris to its list of “urgent threats” to public health apart from four drug-resistant bacterial pathogens (https://www.cdc.gov/drugresistance/pdf/threats-report/candida-auris-508.pdf). Given the importance of C. auris as a health care-associated pathogen, it is concerning that the environmental reservoirs for this pathogen are currently not known. Recently, C. auris was isolated from the salt marsh wetland and sandy beach of Andaman Island, India. However, the link of these isolations to human infection remains to be explored (13). C. auris exhibits relative resistance to widely used quaternary ammonium disinfectants (14), and thus far, only bleach products have been shown to effectively eradicate C. auris from the environment (15).
Rapid testing methods have been developed to identify C. auris from patient samples in the last several years. These included utilization of selective medium for the recovery of C. auris in culture (16), as well as optimization of biochemical (https://www.cdc.gov/fungal/candida-auris/pdf/Testing-algorithm_by-Method_508.pdf) and protein-based databases (http://www.cidrap.umn.edu/news-perspective/2018/04/fda-approves-rapid-diagnostic-test-candida-auris and https://www.rapidmicrobiology.com/news/new-fda-clearance-for-vitek-ms-expanded-id-for-challenging-pathogens) for C. auris identification. Though these methods are excellent, they take considerable time (a few days to weeks) to confirm the identification of C. auris (6). We and others have developed rapid molecular-based assays using various real-time PCR chemistries (17–21). All molecular diagnostics developed thus far for C. auris have relied on DNA, which does not differentiate live from dead C. auris cells. This drawback has hampered environmental surveillance and containment efforts, as the culturing of surveillance samples followed by identification can take anywhere from 4 to 14 days.
The detection of RNA by reverse transcription-quantitative real-time PCR (RT-qPCR) has shown promise for identifying viable microorganisms (22–24). The target used in the present study was the internal transcribed spacer 2 (ITS2) gene of the rRNA, one of the two spacer regions transcribed with the three rRNA subunits (18S, 5.8S, and 28S). The ITS2 gene was chosen because we have extensively validated this target using a real-time PCR assay (17, 18). We explored the transcript product of ribosomal ITS2 RNA as a viability marker using RT-qPCR. ITS2 transcript has essential functions for cell viability, as its presence is necessary for the primary cell metabolism, and deletion of ITS2 from the genome prevents the proper formation of ribosomes, resulting in inhibition of protein synthesis (25–27). ITS2 rRNA is rapidly degraded after transcription (28), and it is unlikely to be detected in nonviable cells. Therefore, the goal of the present investigation was to detect ITS2 transcripts by reverse transcription (ITS2 cDNA) followed by qPCR to determine viable C. auris in the health care environment. There was an excellent concordance between positive RT-qPCR cycle threshold (CT) values and culture for live C. auris from the health care environmental samples. The RT-qPCR assay could be an invaluable tool in surveillance efforts to control the spread of live C. auris in the health care environment.
MATERIALS AND METHODS
Yeast strains and media.
C. auris isolate M5658 South Asia clade I from an outbreak in New York was used for all standardization experiments of the reverse transcription-qPCR (RT-qPCR) assay (17). Additional C. auris isolates from East Asia clade II, South Africa clade III, and South America clade IV and isolates of other Candida species, C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. haemulonii, and C. duobushaemulonii, were used for assay specificity (see Table S1 in the supplemental material) (17, 18). Sabouraud dextrose agar (SDA) slants and plates were used for the culturing of all Candida spp. (6, 21).
RNA extraction.
C. auris cells, grown on SDA for 48 to 72 h, were scraped with a sterile inoculation loop, and the cell suspension was prepared in 1× phosphate-buffered saline (PBS) containing 0.01% bovine serum albumin (BSA). Cells were washed twice with PBS-BSA and resuspended in the same buffer, and an optical density at 530 nm (OD530) of 0.1 was determined. This measurement resulted in 106 CFU/mL of C. auris on the SDA plate (Rokebul Md Anover, personal communication). Serial 10-fold dilutions of C. auris cell suspension prepared in PBS-BSA were centrifuged at 12,500 rpm for 1 min, and RNA from the pellet was extracted using a MasterPure yeast RNA purification kit (Epicenter). In brief, each pellet was suspended in 300 μL of extraction reagent containing 1 μL of 50-μg/μL proteinase K and mixed gently with a vortex shaker (1,000 rpm/min). The mixture was incubated at 70°C for 15 min in the water bath with intermittent mixing every 5 min. Following incubation, samples were placed in ice for 5 min, and then 175 μL of MPC protein precipitation reagent was added to 300 μL of lysed samples. The mixture was vortexed briefly for 10 s and then centrifuged at 12,500 rpm for 10 min at 4°C. Supernatant was transferred to a clean tube, and the pellet was discarded. The RNA in the supernatant was precipitated by adding 500 μL of isopropanol, inverting the tube 30 to 40 times, and centrifuging it at 12,500 rpm at 4°C. Isopropanol was removed without dislodging the pellet. The pellet was washed twice with 70% ethanol, and the RNA was resuspended in 40 μL of Tris EDTA (TE) buffer. Any DNA contamination in the extracted RNA was removed by treating each sample with 5 μL of Turbo DNase buffer and 1 μL of Turbo DNase enzyme (Thermo Fisher Scientific). Samples were mixed gently by flicking the tube, followed by incubation at 37°C for 30 min in the water bath. Following incubation, 5 μL of DNase inactivation reagent was added, and the samples were incubated at room temperature for 5 min with intermittent flicking. The samples were centrifuged at 12,500 rpm for 5 min at room temperature, and then the liquid was transferred to a new tube and the pellet was discarded. All purified RNA was stored at −20°C until further testing. RNA from other Candida species was also extracted using the same procedure.
RNA was also extracted from environmental samples (sponges) collected from various health care facilities. Each sample was placed in a Whirl-Pak homogenizer blender filter bag (Whirl-Pak, Madison, WI, USA) containing 45 mL of PBS containing 0.01% Tween 80 (PBS-T80). The bags were gently mixed in a Stomacher 400 circulator (Laboratory Supply Network, Inc., Atkinson, NH, USA) at 260 rpm for 1 min to remove the content of the sponge in the liquid, The liquid was transferred into a 50-mL conical tube and centrifuged at 4,000 rpm for 5 min, and the supernatant was decanted, leaving about 3 mL of liquid at the bottom of the tube. The 3 mL of liquid was vortexed briefly, and from this, 1-mL aliquots of surveillance samples were drawn and spiked with 10-fold dilution series of C. auris, and then RNA was extracted as described above. RNA was also extracted from unspiked environmental samples.
RT-qPCR assay.
Ultra Plex 1-step ToughMix (Quanta Bio, Beverly, MA, USA), which is a ready-to-use 4× concentrated master mix for 1-step reverse transcription and real-time quantitative PCR (RT-qPCR) of RNA templates using probe-based detection methods (e.g., TaqMan 5′-hydrolysis probes) on ABI7500 (Applied Biosystems), was used. In this system, the first-strand cDNA synthesis and PCR amplification were carried out in the same tube without opening between the procedures. The PCR amplification was carried out in 20 μL final volume containing 5 μL of RNA template, 1 μL (500 nM) each of ITS2 forward (V2424F [CAURF], 5′-CAGACGTGAATCATCGAATCT-3′), and reverse (V2426 (CAURR), 5′-TTTCGTGCAAGCTGTAATTT-3′), primers, 0.8 μL (100 nM) of ITS2 (V2425P (CAURP), 5′-/56-carboxyfluorescein (FAM)/AATCTTCGC/ZEN/GGTGGCGTTGCATTCA/3IABkFQ/-3′) probe, 5 μL of Ultra Plex 1-step ToughMix, and 7.2 μL of nucleic acid-free water. All PCR runs also included 5 μL of positive extraction (C. auris M5658; 103 CFU/50 μL) and positive amplification (C. auris M5658, 0.02 pg/μL), 5 μL of negative extraction (reagents only), and negative amplification (nuclease-free water) control. All experiments with internal positive control (VetMAX XENO) were prepared by adding 1.2 μL of XENO control to the master mix, and the volume of water in the master mix was reduced to 6.0 μL. The probe in the internal control was tagged with VIC dye.
The RT-qPCRs were carried out in the ABI 7500 FAST (Applied Biosystems) instrument starting with cDNA synthesis at 50°C for 10 min, followed by initial denaturation at 95°C for 3 min and qPCR cycling (45 cycles) conditions of denaturation at 95°C for 3 s and annealing at 60°C for 30 s. The cycle threshold (CT) was determined by the instrument. The assay sensitivity and specificity were analyzed in triplicate, while assay reproducibility and retrospective studies were done in duplicate. The coefficient of efficiency (E) was calculated using the formula E = 10(−1/slope) − 1. CT values of ≤40 were considered positive, and CT values of >40 were considered negative. All RNA extracted samples were also assessed for DNA contamination by real-time PCR assay as described previously (17).
RT-qPCR assay sensitivity, reproducibility, and specificity.
C. auris isolate M5658 South Asia clade I from an outbreak in New York (17) was used to assess the analytical sensitivity of the RT-qPCR assay. The analytical sensitivity was determined in 10-fold serial dilutions of C. auris cells in PBS-BSA and in environmental sponge matrix. In brief, environmental sponge samples negative for C. auris DNA by earlier established real-time PCR assay (17), and also negative for C. auris by culture, were pooled and spiked with various concentrations of live C. auris cells. All spiked samples were processed for RNA extraction, and 5 μL of the extracted RNA was used in the RT-qPCR assay in triplicate.
Since primers and probe were assessed extensively for assay specificity in our previous investigation (17), only a small panel of Candida spp. was tested in the present investigation. In brief, 5 μL of extracted RNA from each of the C. auris clades I to IV and other Candida spp., including C. haemulonii, C. duobushaemulonii, C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis, was run in an RT-qPCR assay in triplicate. The assay reproducibility was assessed by spiking pooled negative surveillance samples with various concentrations of live C. auris, bleach-killed C. auris, and other live Candida spp., followed by RNA extraction and RT-qPCR assay.
Retrospective analysis of environmental surveillance samples.
A total of 33 surveillance samples collected in the year 2020, stored at 4°C, and 24 of the 33 samples positive by earlier real-time PCR assay (17) were part of this investigation. A 1-mL aliquot of each sample was processed for RNA extraction as described above, followed by RT-qPCR assay.
Heat, bleach, and ethanol inactivation of C. auris.
C. auris (M5658) cells grown on an SDA plate for 72 h were scraped and suspended in PBS-BSA, and the OD530 of 0.1 (equivalent to 106 CFU/mL) was determined. Several 1-mL aliquots were incubated at 90°C for 1 h with shaking at 400 rpm in the Eppendorf ThermoMixer C. RNA was extracted from the sample just after incubation, and then 30 min, 1 h, 2 h, 4 h, 24 h, and 48 h postincubation at room temperature followed by RNA extraction and RT-qPCR. For bleach treatment, each 1-mL aliquot of C. auris cells was washed with PBS-BSA and resuspended in 900 μL of PBS-BSA, and 100 μL of bleach from a stock bottle was added to get a final concentration of 10% bleach per tube. For ethanol treatment, each C. auris cell suspension aliquot was washed with PBS-BSA and resuspended in 300 μL of PBS-BSA. To get a final concentration of 70% ethanol, 700 μL of 100% ethanol was added to each tube. Bleach and ethanol treatments were carried out at room temperature for 1, 3, 5, 10, and 30 min, followed by washing with PBS-BSA, RNA extraction, and RT-qPCR assay. Another set of C. auris cells were treated with 70% ethanol for 10 min followed by washing cells with PBS-BSA and incubation at room temperature for 30 min to 48 h. The cell viability of each treatment was assessed by plating C. auris cell suspension (106 CFU/mL) on an SDA plate and incubating it at 30°C for 96 h. One aliquot of C. auris cells (106CFU/mL) without any treatment was also processed for RNA extraction followed by RT-qPCR, which served as the control in various experiments.
Statistical analysis.
The GraphPad Prism 8.0 software for macOS was used for calculation of the mean, standard deviation, and percent coefficient of variance (CV) for CT values generated for assay sensitivity and reproducibility. Student’s t test was used for the analysis of means and variance, and a P value of <0.05 was considered statistically significant.
RESULTS
RT-qPCR assay sensitivity, specificity, and reproducibility.
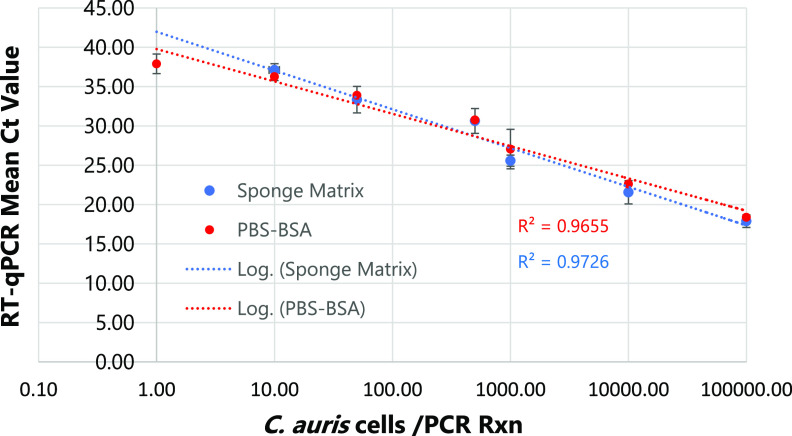
The RT-qPCR assay was linear over 5 orders of magnitude, and the limit of detection of the assay was 10 CFU/PCR in PBS-BSA and sponge matrix (Fig. 1). The assay was highly specific, as no PCR amplification was detected for Candida species other than C. auris (Table S1). The assay was highly reproducible when blinded surveillance samples spiked with either live or bleach-killed C. auris or live Candida spp. other than C. auris strains were analyzed. Of 17 environmental samples spiked with low to high numbers of live C. auris cells (102 to 105), 15 yielded positive results for C. auris by RT-qPCR, while all nonspiked as well as spiked environmental samples with bleach-killed C. auris and other live Candida spp. were negative, confirming assay reproducibility and specificity (Table 1). Using the culture method as the gold standard, the accuracy of the RT-qPCR assay for the detection of live C. auris from surveillance samples was 98%, with a positive predictive value and negative predictive value of 100% and 87%, respectively (Table 2).
FIG 1.
Sensitivity of RT-qPCR assay. PBS-BSA and environmental sponge samples were spiked with 10-fold dilution series of C. auris cells followed by RNA extraction and RT-qPCR run in triplicate. The assay was linear over 5 orders of magnitude with an assay sensitivity of 10 C. auris CFU/PCR.
TABLE 1.
C. auris RT-qPCR assay reproducibility using blinded panels
| Sample no. | Yeast spp. spiked in surveillance sample | No. of yeast cells spiked per sample | No. of yeast cells/PCR reaction | Live or dead yeast | RT-qPCR |
Internal control mean CT | Final interpretation | ||
|---|---|---|---|---|---|---|---|---|---|
| CT 1 | CT 2 | Mean CT | |||||||
| 1 | C. albicans | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 32.26 | Negative |
| 2 | C. auris | 105 | 104 | Live | 23.26 | 23.89 | 23.62 | 32.42 | Positive |
| 3 | C. auris | 105 | 104 | Live | 23.31 | 23.99 | 23.65 | 32.81 | Positive |
| 4 | C. auris | 104 | 103 | Live | 28.32 | 28.11 | 28.21 | 32.36 | Positive |
| 5 | C. auris | 103 | 102 | Live | 33.17 | 32.96 | 33.06 | 32.42 | Positive |
| 6 | C. albicans | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 32.52 | Negative |
| 7 | C. auris | 103 | 102 | Live | 32.77 | 28.9 | 30.83 | 33.41 | Positive |
| 8 | None | 0.0 | 0.0 | 0.0 | 32.58 | Negative | |||
| 9 | C. auris | 104 | 103 | Live | 27.08 | 28.9 | 28.0 | 32.45 | Positive |
| 10 | C. auris | 105 | 104 | Dead | 0.0 | 0.0 | 0.0 | 32.44 | Negative |
| 11 | C. auris | 105 | 104 | Dead | 0.0 | 0.0 | 0.0 | 32.93 | Negative |
| 12 | C. auris | 104 | 104 | Dead | 0.0 | 0.0 | 0.0 | 32.02 | Negative |
| 13 | None | 0.0 | 0.0 | 0.0 | 32.33 | Negative | |||
| 14 | C. glabrata | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 32.55 | Negative |
| 15 | C. auris | 105 | 104 | Dead | 0.0 | 0.0 | 0.0 | 33.33 | Negative |
| 16 | C. auris | 102 | 101 | Live | 35.74 | 37.62 | 36.68 | 33.98 | Positive |
| 17 | C. auris | 105 | 104 | Live | 24.64 | 24.93 | 24.75 | 32.40 | Positive |
| 18 | C. auris | 104 | 103 | Live | 28.63 | 24.94 | 26.78 | 32.12 | Positive |
| 19 | C. auris | 102 | 101 | Live | 37.98 | 37.00 | 37.48 | 33.20 | Positive |
| 20 | C. auris | 102 | 101 | Live | 0.0 | 0.0 | 0.0 | 32.76 | Negativea |
| 21 | C. auris | 102 | 101 | Live | 38.0 | 40.72 | 39.32 | 32.76 | Positive |
| 22 | C. auris | 102 | 101 | Live | 0.0 | 0.0 | 0.0 | 33.15 | Negativea |
| 23 | None | 0.0 | 0.0 | 0.0 | 33.87 | Negative | |||
| 24 | C. auris | 105 | 104 | Live | 24.71 | 25.5 | 25.10 | 32.42 | Positive |
| 25 | C. auris | 104 | 103 | Live | 26.99 | 25.59 | 26.29 | 32.98 | Positive |
| 26 | C. haemulonii | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 32.76 | Negative |
| 27 | C. duobushaemulonii | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 32.75 | Negative |
| 28 | C. haemulonii | 105 | 104 | Live | 0.0 | 0.0 | 0.0 | 34.40 | Negative |
| 29 | C. auris | 105 | 104 | Live | 22.5 | 22.3 | 22.4 | 32.62 | Positive |
| 30 | C. auris | 105 | 104 | Live | 22.94 | 22.76 | 22.85 | 33.24 | Positive |
| NEC | 0.0 | 0.0 | 0.0 | Negative | |||||
| XENO | 31.57 | Positive | |||||||
| NTC | 0.0 | 0.0 | 0.0 | Negative | |||||
| PAC | C. auris | 104 | 103 | Live | 28.01 | 28.16 | 28.08 | Positive | |
Samples showing discrepant results.
TABLE 2.
Comparison of culture and RT-qPCR assay of spiked blinded surveillance samplesa
| RT-qPCR | No. of surveillance samples spiked with indicated culture result |
Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Live C. auris (positive) | Live other Candida spp. (positive) | Dead C. auris (negative) | No organism (negative) | ||||||
| No. positive | 15 | 0 | 0 | 0 | 93 | 88 | 100 | 100 | 87 |
| No. negative | 2 | 6b | 4b | 3b | |||||
PPV, positive predictive value; NPV, negative predictive value.
Samples spiked with dead C. auris or other organisms and those spiked with no organisms are considered true-negative samples in this analysis; thus, the number of true-negative samples is 13 in the subsequent calculations.
Stability of ITS2 RNA in heat-, ethanol-, and bleach-killed C. auris.
The effective environmental disinfectants for C. auris are Clorox-based products (29). Generally recommended cleaning practices for the environmental surfaces are wiping with 10% bleach followed by 70% ethanol (https://www.cdc.gov/fungal/candida-auris/c-auris-lab-safety.html). Since decontamination of linen products in the health care facilities requires high temperatures, we also included a high-temperature treatment at 90°C. The bleach treatment abolished RNA, resulting in no CT values by RT-qPCR, while ethanol treatment of C. auris cells for 1 min to 30 min did not degrade RNA, resulting in CT values similar to those of untreated C. auris cells (Fig. 2A). In a second set of experiments, C. auris cells were treated with 70% ethanol for 10 min at room temperature, followed by washing and then incubation for a prolonged period (30 min to 48 h) at room temperature. The ethanol did not degrade RNA, as evident from no change in CT values compared to untreated C. auris cells (Fig. 2B). RNA was also stable with heat treatment, as the CT remained unchanged until 24 h postincubation with significant degradation (P < 0.05) observed at 48 h postincubation (Fig. 2B). The bleach, ethanol, and heat treatment resulted in no growth of C. auris in culture plates (data not shown).
FIG 2.
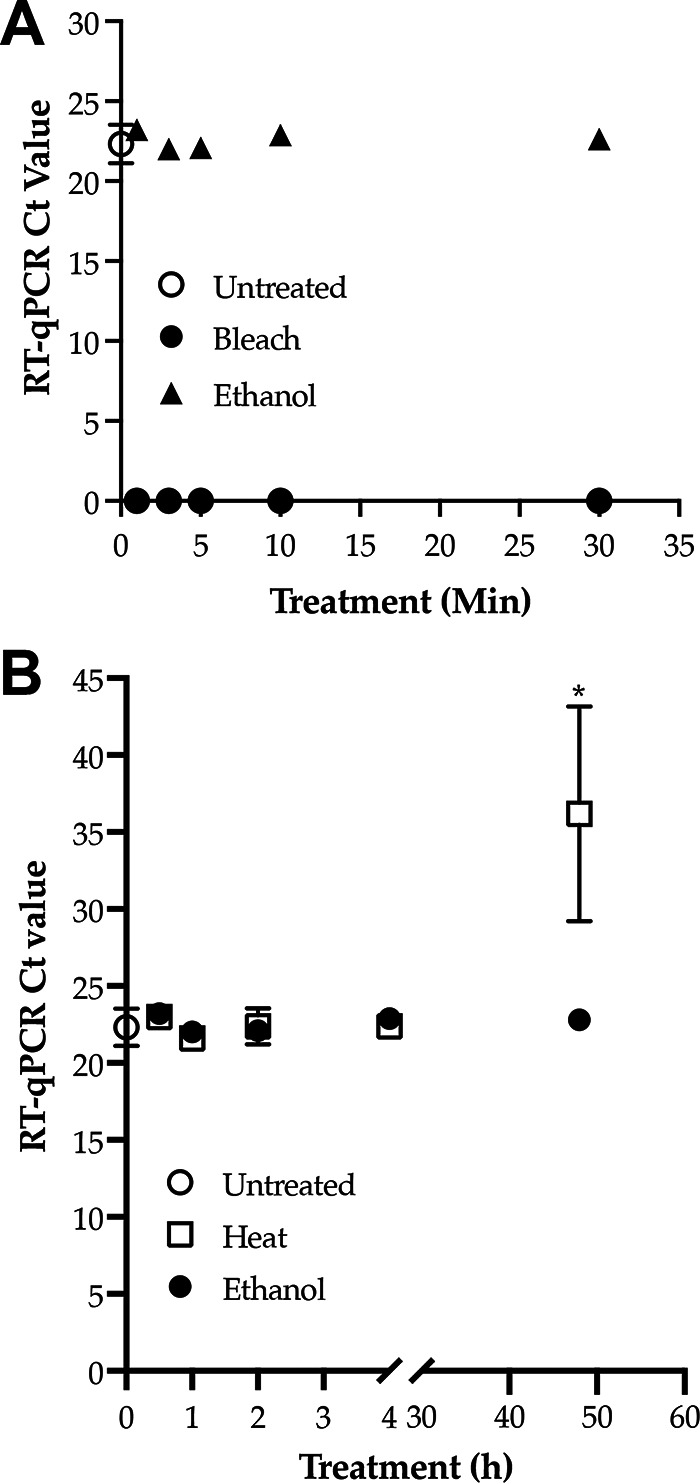
C. auris RNA stability following treatment with bleach, ethanol, and heat. Candida auris cells grown in SDA for 72 h at 30°C were scrapped and suspended in PBS-BSA, and an OD530 of 0.1 was determined. Each 1-mL aliquot of 0.1 OD (equivalent to 106 CFU/mL) cells was used as untreated, which served as the control, and treated with 10% bleach, 70% ethanol, and heat at 90°C. RNA was extracted and RT-qPCR was run in triplicate. (A) Treatment with bleach and ethanol for different time points. Bleach treatment of C. auris of as little as 1 min completely abolished ITS2 RNA, resulting in no CT values. In contrast, ethanol treatment of C. auris for even 30 min had no impact on ITS2 RNA. CT values of ethanol-treated cells were comparable to the CT values of the untreated C. auris cells. (B) Heat treatment for 1 h followed by incubation at room temperature for 30 min to 48 h; ethanol treatment for 10 min followed by washing in PBS-BSA and incubation at room temperature for 30 min to 48 h. Ethanol did not alter ITS2 RNA after prolonged incubation, as evidenced by comparable CT values for ethanol and untreated C. auris cells. In contrast, heat treatment substantially degraded ITS RNA (P < 0.05) following 48 h postincubation (asterisk) compared to the ethanol-treated or untreated control cells.
Retrospective analysis of environmental surveillance samples.
Of 33 previously analyzed samples, 24 were positive for C. auris DNA by our earlier established RT-PCR assay (17), while they were all negative by culture. When these samples were subjected to RNA extraction followed by RT-qPCR assay, all of them were negative for ITS2 RNA, confirming culture results (Table 3).
TABLE 3.
Retrospective analysis of environmental samples from the year 2020
| Sample no. | Culture | Real-time PCR mean CT value | RT-qPCR |
Final interpretation | |
|---|---|---|---|---|---|
| CT 1 | CT 2 | ||||
| 1 | Negative | 35.23 | 0.0 | 0.0 | Negative |
| 2 | Negative | 33.71 | 0.0 | 0.0 | Negative |
| 3 | Negative | 37.26 | 0.0 | 0.0 | Negative |
| 4 | Negative | 32.17 | 0.0 | 0.0 | Negative |
| 5 | Negative | 34.38 | 0.0 | 0.0 | Negative |
| 6 | Negative | 31.62 | 0.0 | 0.0 | Negative |
| 7 | Negative | 32.65 | 0.0 | 0.0 | Negative |
| 8 | Negative | 32.14 | 0.0 | 0.0 | Negative |
| 9 | Negative | 35.58 | 0.0 | 0.0 | Negative |
| 10 | Negative | 37.08 | 0.0 | 0.0 | Negative |
| 11 | Negative | 32.62 | 0.0 | 0.0 | Negative |
| 12 | Negative | 36.32 | 0.0 | 0.0 | Negative |
| 13 | Negative | 33.54 | 0.0 | 0.0 | Negative |
| 14 | Negative | 35.51 | 0.0 | 0.0 | Negative |
| 15 | Negative | 34.33 | 0.0 | 0.0 | Negative |
| 16 | Negative | 28.43 | 0.0 | 0.0 | Negative |
| 17 | Negative | 35.73 | 0.0 | 0.0 | Negative |
| 18 | Negative | 36.26 | 0.0 | 0.0 | Negative |
| 19 | Negative | 36.38 | 0.0 | 0.0 | Negative |
| 20 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 21 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 22 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 23 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 24 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 25 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 26 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 27 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 28 | Negative | 0.0 | 0.0 | 0.0 | Negative |
| 29 | Negative | 32.20 | 0.0 | 0.0 | Negative |
| 30 | Negative | 35.4 | 0.0 | 0.0 | Negative |
| 31 | Negative | 37.07 | 0.0 | 0.0 | Negative |
| 32 | Negative | 35.21 | 0.0 | 0.0 | Negative |
| 33 | Negative | 34.37 | 0.0 | 0.0 | Negative |
| NEC | NAa | 0.0 | 0.0 | 0.0 | Negative |
| XENO | NA | NA | 31.57 | 31.56 | Positive |
| NTC | NA | 0.0 | 0.0 | 0.0 | Negative |
| PEC | Positive | 29.46 | 29.32 | 28.46 | Positive |
NA, not applicable.
DISCUSSION
We developed the new C. auris RT-qPCR assay based on rRNA as a cell viability marker, as RNA is less stable than DNA after cellular death (22–24). The selection of ITS RNA allowed us to recycle proven primers and probes from a previous C. auris real-time PCR assay (17). The numerous replicates obtained for RT-qPCR assay sensitivity and the small standard error among these replicates proved that the assay was highly robust and reproducible. The assay’s detection limit was 10 CFU of C. auris per RT-qPCR, confirming the high sensitivity. The RT-qPCR assay was highly specific, as it did not cross-react with closely or distantly related Candida spp.
C. auris causes prolonged contamination of the health care environment (16). In the absence of a known ecological niche, the presence of C. auris on inanimate objects in the health care setting could serve as a source for new infection (3, 5, 6). Therefore, infection control requires extensive decontamination of floors and objects in the vicinity of the C. auris-positive patients (30). The preferred decontamination agents have sodium hypochlorite (bleach) as an active ingredient (https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html). Until now, we and others have heavily relied on the rapid DNA-based real-time PCR assays to assess the effectiveness of the decontamination procedures since culture results take much longer (4 to 14 days). However, there is a low concordance between C. auris DNA-positive and culture-positive results for environmental samples (5). Apparently, the results of the real-time PCR assay are skewed by the presence of C. auris DNA from live, dead, and growth-defective C. auris (5). Thus, laboratories that support ongoing decontamination efforts need a rapid test for the environmental samples that shows good concordance with the culture results.
In the present study, decontamination was simulated by treatment of C. auris with 10% bleach, 70% ethanol, and 90°C heat. The rationale for these three conditions was that (i) the products with the active ingredient, sodium hypochlorite (bleach) (https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants#candida-auris), have proved to be effective in decontamination of C. auris from health care environments, (ii) the use of 70% ethanol following bleach treatment is standard practice to remove residual bleach from inanimate objects as part of safety protocols for staff and for the sustainability and durability of health care objects, and (iii) heat-killing at high temperature is a requirement for cleaning linens in health care facilities facing C. auris outbreak (https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html). Bleach treatment was effective, as no C. auris RNA was detected by RT-qPCR from cells 1 min posttreatment. Surprisingly, C. auris RNA was stable in the ethanol-killed cells, but it was significantly degraded in the heat-killed cells at 48 h post-cell death. Since the incubation conditions after heat or ethanol treatment were identical, the difference observed might be related to the effect of heat or ethanol treatment on RNA breakdown in dead cells. Results similar to our observations were reported for both eukaryotic (Saccharomyces cerevisiae) and prokaryotic (Escherichia coli) organisms (22, 23). The excellent concordance observed between live- and bleach-treated C. auris and RT-qPCR CT values for spiked environmental samples affirms the current choice of sodium hypochlorite (bleach) products for the decontamination of the health care environment (15). Furthermore, the retrospective analyses of 33 environmental samples showed an excellent correlation between negative RT-qPCR values and those of negative culture results.
There are some obvious limitations of the present study. As RNA extraction is more labor-intensive than crude DNA extraction, the new assay will not be as high-throughput as our previous assays (17, 18). Quantitative PCR systems based on reverse transcription have mostly used mRNA as the template, whereas our primers were designed to amplify rRNA (ITS2). Detection of mRNA might be a better technology, as its shelf life is shorter than that of RNA. Despite their potential advantages, mRNA-based approaches have proved difficult because of the complexity of the methods, the practical problems of extracting detectable levels of intact mRNA from small numbers of cells, and a lack of basic information about the significance of detecting mRNA in the stressed cells. As ITS2 RNA is stable in C. auris cells treated with heat or ethanol, our test will only work with surveillance samples obtained after recommended decontamination with sodium hypochlorite-based agents. Lastly, our retrospective evaluation of environmental samples only showed correlation between RNA and culture for the negative samples. The RNA extraction using an Epicenter kit followed by removal of residual DNA by Turbo DNase proved to be excellent for isolation of pure RNA. DNA contamination from as high as 106 C. auris cells was almost nil to a minimal level (CT, >40.0) as determined by real-time PCR assay.
In conclusion, the new C. auris RT-qPCR is a fast, direct (without culture), sensitive, and reliable technique for the detection of live C. auris cells from surveillance samples. This newly developed assay can strengthen ongoing decontamination efforts for the effective control and prevention of C. auris in the health care environment.
ACKNOWLEDGMENTS
We thank Wadsworth Center (WC) Tissue Culture & Media Cores for providing various media for culture of Candida spp. We also thank Kimberlee McClive-Reed for editing the manuscript.
Bryanna Freitas was partially supported by the Wadsworth Center New York State Department of Health (WC-NYSDOH) Fellowship Program. This work was supported in part with funds from WC-NYSDOH and Centers for Disease Control and Prevention (CDC) grant number NU50CK000516. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NYSDOH or the CDC.
S.C. conceived the study, supervised experiments and data interpretation, and wrote the manuscript. B.L.F. performed the majority of the experiments and prepared the graphs and tables. L.L. assessed the experimental and control reagents for RT-qPCR assay, supervised part of B.L.F.’s work, and tabulated previously analyzed data for the retrospective study. V.C. contributed to the study design and data interpretation and edited the draft manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Sudha Chaturvedi, Email: Sudha.Chaturvedi@health.ny.gov.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 4.Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, Griffiths D, George S, Butcher L, Morgan M, Newnham R, Sunderland M, Clarke T, Foster D, Hoffman P, Borman AM, Johnson EM, Moore G, Brown CS, Walker AS, Peto TEA, Crook DW, Jeffery KJM. 2018. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med 379:1322–1331. 10.1056/NEJMoa1714373. [DOI] [PubMed] [Google Scholar]
- 5.Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, Greenko J, Fernandez R, Kallen A, Vallabhaneni S, Haley V, Hutton B, Blog D, Lutterloh E, Zucker H, Candida auris Investigation Workgroup. 2018. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 24:1816–1824. 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, O’Brien B, Leach L, Clarke A, Bates M, Adams E, Ostrowsky B, Quinn M, Dufort E, Southwick K, Erazo R, Haley VB, Bucher C, Chaturvedi V, Limberger RJ, Blog D, Lutterloh E, Chaturvedi S. 2020. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol 58:e01503-19. 10.1128/JCM.01503-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron G, Bloch D, Murray K, Kratz M, Parton H, Ackelsberg J, Antwi M, Del Rosso P, Dorsinville M, Kubinson H, Lash M, Rand S, Adams E, Zhu Y, Erazo R, Chaturvedi S, Weiss D. 2020. Candida auris colonization after discharge to a community setting: New York City, 2017–2019. Open Forum Infect Dis 8:ofaa6209. 10.1093/ofid/ofaa620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, C. auris Investigation Work Group. 2020. Candida auris isolates resistant to three classes of antifungal medications: New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien B, Liang J, Chaturvedi S, Jacobs JL, Chaturvedi V. 2020. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe 1:e193–e194. 10.1016/S2666-5247(20)30090-2. [DOI] [PubMed] [Google Scholar]
- 10.Lyman M, Forsberg K, Reuben J, Dang T, Free R, Seagle EE, Sexton DJ, Soda E, Jones H, Hawkins D, Anderson A, Bassett J, Lockhart SR, Merengwa E, Iyengar P, Jackson BR, Chiller T. 2021. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities: Texas and the District of Columbia, January-April 2021. MMWR Morb Mortal Wkly Rep 70:1022–1023. 10.15585/mmwr.mm7029a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piedrahita CT, Cadnum JL, Jencson AL, Shaikh AA, Ghannoum MA, Donskey CJ. 2017. Environmental surfaces in healthcare facilities are a potential source for transmission of Candida auris and other Candida species. Infect Control Hosp Epidemiol 38:1107–1109. 10.1017/ice.2017.127. [DOI] [PubMed] [Google Scholar]
- 12.Pacilli M, Kerins JL, Clegg WJ, Walblay KA, Adil H, Kemble SK, Xydis S, McPherson TD, Lin MY, Hayden MK, Froilan MC, Soda E, Tang AS, Valley A, Forsberg K, Gable P, Moulton-Meissner H, Sexton DJ, Jacobs Slifka KM, Vallabhaneni S, Walters MS, Black SR. 2020. Regional emergence of Candida auris in Chicago and lessons learned from intensive follow-up at 1 ventilator-capable skilled nursing facility. Clin Infect Dis 71:e718–e725. 10.1093/cid/ciaa435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora P, Singh P, Wang Y, Yadav A, Pawar K, Singh A, Padmavati G, Xu J, Chowdhary A. 2021. Environmental Isolation of Candida auris from the coastal wetlands of Andaman Islands, India. mBio 12:e03181-20. 10.1128/mBio.03181-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadnum JL, Shaikh AA, Piedrahita CT, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. 2017. Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol 38:1240–1243. 10.1017/ice.2017.162. [DOI] [PubMed] [Google Scholar]
- 15.Ku TSN, Walraven CJ, Lee SA. 2018. Candida auris: disinfectants and implications for infection control. Front Microbiol 9:726. 10.3389/fmicb.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, Litvintseva AP. 2017. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 55:2996–3005. 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leach L, Zhu Y, Chaturvedi S. 2018. Development and validation of a real-time PCR assay for rapid detection of Candida auris from surveillance samples. J Clin Microbiol 56:e01223-17. 10.1128/JCM.01223-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach L, Russell A, Zhu Y, Chaturvedi S, Chaturvedi V. 2019. A rapid and automated sample-to-result Candida auris real-time PCR assay for high-throughput testing of surveillance samples with the BD Max Open system. J Clin Microbiol 57:e00630-19. 10.1128/JCM.00630-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kordalewska M, Zhao Y, Lockhart SR, Chowdhary A, Berrio I, Perlin DS. 2017. Rapid and accurate molecular identification of the emerging multidrug-resistant pathogen Candida auris. J Clin Microbiol 55:2445–2452. 10.1128/JCM.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima A, Widen R, Vestal G, Uy D, Silbert S. 2019. A TaqMan probe-based real-time PCR assay for the rapid identification of the emerging multidrug-resistant pathogen Candida auris on the BD Max System. J Clin Microbiol 57:e01604-18. 10.1128/JCM.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis EK, Chaturvedi S, Chaturvedi V. 2021. So many diagnostic tests, so little time: review and preview of Candida auris testing in clinical and public health laboratories. Front Microbiol 12:757835. 10.3389/fmicb.2021.757835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hierro N, Esteve-Zarzoso B, González A, Mas A, Guillamón JM. 2006. Real-time quantitative PCR (QPCR) and reverse transcription-QPCR for detection and enumeration of total yeasts in wine. Appl Environ Microbiol 72:7148–7155. 10.1128/AEM.00388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan GE, Masters CI, Shallcross JA, MacKey BM. 1998. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 64:1313–1318. 10.1128/AEM.64.4.1313-1318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keer JT, Birch L. 2003. Molecular methods for the assessment of bacterial viability. J Microbiol Methods 53:175–183. 10.1016/s0167-7012(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 25.Good L, Intine RV, Nazar RN. 1997. Interdependence in the processing of ribosomal RNAs in Schizosaccharomyces pombe. J Mol Biol 273:782–788. 10.1006/jmbi.1997.1351. [DOI] [PubMed] [Google Scholar]
- 26.Musters W, Boon K, van der Sande CA, van Heerikhuizen H, Planta RJ. 1990. Functional analysis of transcribed spacers of yeast ribosomal DNA. EMBO J 9:3989–3996. 10.1002/j.1460-2075.1990.tb07620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konikkat S, Woolford JL Jr. 2017. Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast. Biochem J 474:195–214. 10.1042/BCJ20160516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milligan L, Decourty L, Saveanu C, Rappsilber J, Ceulemans H, Jacquier A, Tollervey D. 2008. A yeast exosome cofactor, Mpp6, functions in RNA surveillance and in the degradation of noncoding RNA transcripts. Mol Cell Biol 28:5446–5457. 10.1128/MCB.00463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE, Weber DJ. 2019. Susceptibility of Candida auris and Candida albicans to 21 germicides used in healthcare facilities. Infect Control Hosp Epidemiol 40:380–382. 10.1017/ice.2019.1. [DOI] [PubMed] [Google Scholar]
- 30.Sabino R, Verissimo C, Pereira AA, Antunes F. 2020. Candida auris, an agent of hospital-associated outbreaks: which challenging issues do we need to have in mind? Microorganisms 8:181. 10.3390/microorganisms8020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.00779-21-s0001.xlsx, XLSX file, 0.01 MB (9.9KB, xlsx)