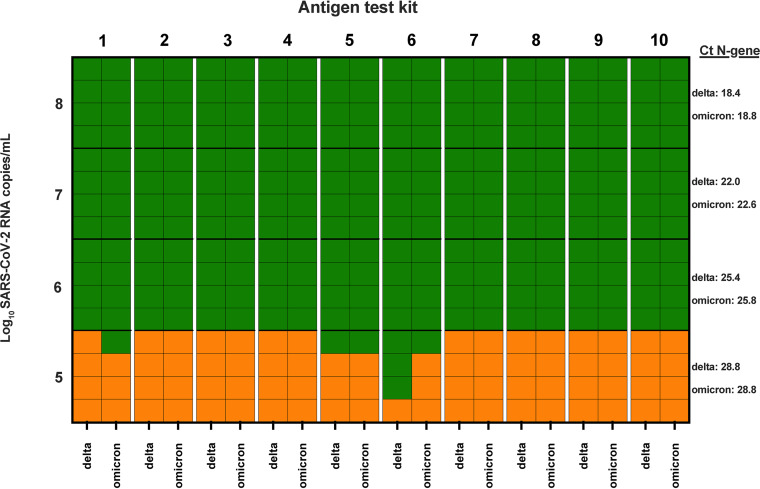
FIG 1.
Analytical sensitivities of lateral flow devices against SARS-CoV-2 Delta and Omicron variants. Ten lateral flow devices were tested against 10-fold dilutions (1:100 to 1:100,000) of SARS-CoV-2 Delta and Omicron variants in quadruplicate. Green boxes indicate where SARS-CoV-2 antigen was detected for a single replicate, and orange boxes indicate where a negative result was observed. Mean Ct values from three replicates for each dilution were calculated using in-house RT-PCR for the N gene. The registered test names and manufacturers of the lateral flow devices included were as follows: (1) PanBio COVID-19 Ag rapid test device (nasal), Abbott Rapid Diagnostics Jena GmbH (Germany); (2) NowCheck COVID-19 antigen test, BioNote Inc. (Republic of Korea); (3) Roche SARS-CoV-2 rapid antigen test, SD Biosensor Inc. (Republic of Korea); (4) Standard Q COVID-19 Ag test, SD Biosensor Inc. (Republic of Korea); (5) SureScreen Diagnostics COVID-19 antigen rapid test cassette, BTNX Inc. (Canada); (6) VivaDiag SARS-CoV-2 Ag rapid test, VivaChek Biotech (Hangzhou) Co. Ltd. (China); (7) Wantai SARS-CoV-2 Ag rapid test (colloidal gold), Beijing Wantai Biological Pharmacy Enterprise Co. Ltd. (China); (8) Testsea SARS-CoV-2 antigen test kit, Hangzhou Testsea Biotechnology Co. Ltd. (China); (9) InnoScreen COVID-19 antigen rapid test device, Innovation Scientific Pty. Ltd. (Australia); and (10) LYHER novel coronavirus (COVID-19) antigen test kit (colloidal gold), Hangzhou Laihe Biotech Co. Ltd. (China).