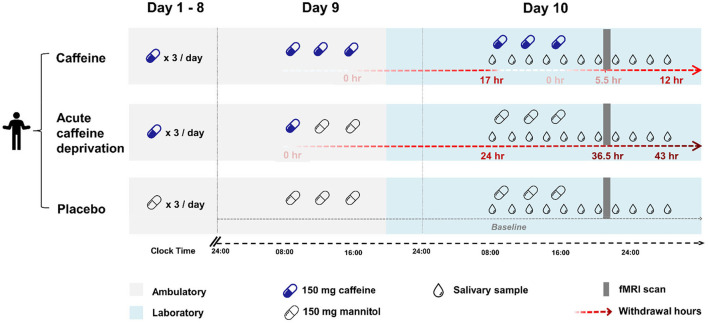
Figure 1.
An overview of the study design. Each condition consisted of 9 ambulatory days (grey shading) and a subsequent 21-h laboratory measurement starting in the evening of day 9 (blue shading). The timing of caffeine or placebo intake is indicated by capsules (blue: caffeine, transparent: placebo), the timing of saliva collection to measure caffeine and paraxanthine levels is indicated by drops. Top: Caffeine condition. Caffeine capsules (150 mg caffeine) × 3 times/ day (at 45 min, 4 h, and 8 h after waking up) were administered throughout the 10 days. Saliva was collected repeatedly during the laboratory stay from right before the first capsule until 12 h after the latest caffeine intake. The MRI scan (grey bar) took place at 5.5 h after the latest caffeine intake. Middle: Acute caffeine deprivation. Caffeine capsules (150 mg caffeine) × 3 times/ day was administered for 8 days, followed by a switch to placebo capsules on day 9. Saliva was collected between 24 and 43 h after the latest caffeine intake. The MRI scan was scheduled at 36.5 h after the latest caffeine intake, a time window when strong withdrawal symptoms are often expressed (25). Bottom: Placebo condition. Placebo capsules (150 mg mannitol) × 3 times/ day were administered throughout the ambulatory and laboratory days. Saliva was collected at baseline through the entire laboratory phase at the corresponding time points as in the other two conditions. The MRI scan was scheduled at the same time of day as in the other two conditions.