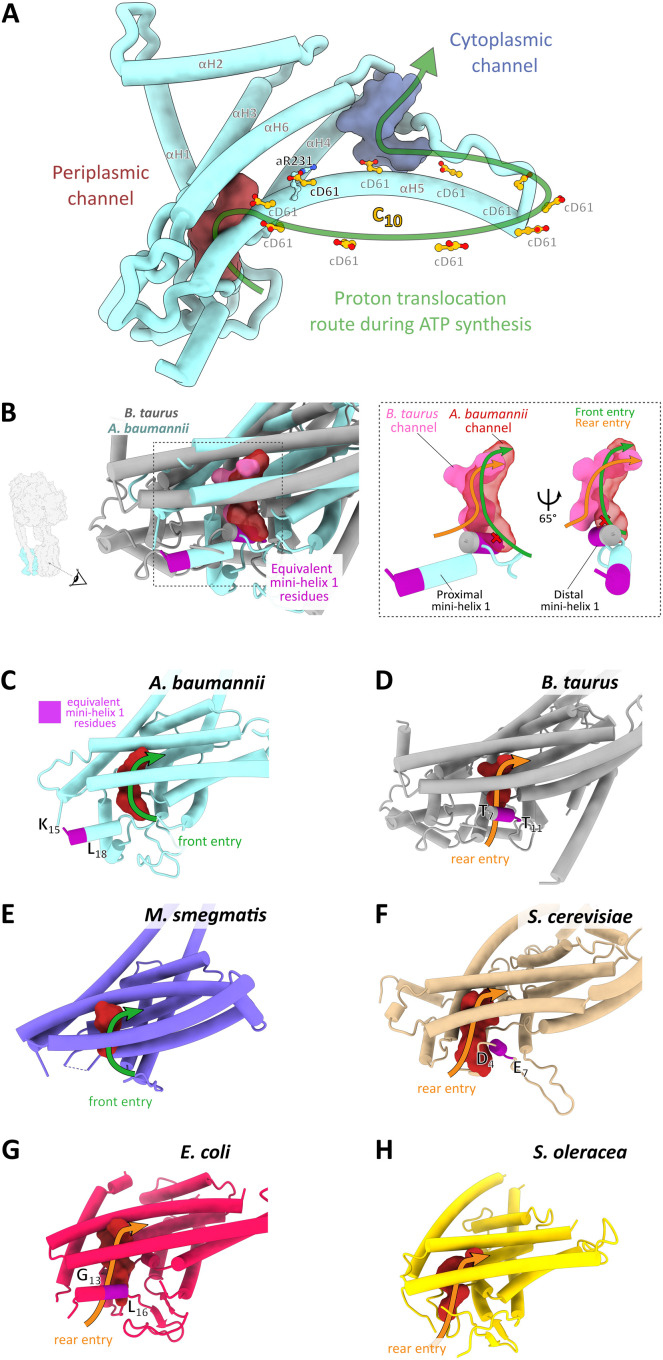
Fig. 3. Structure of the Fo motor and an Acinetobacter-specific proton entry channel site.
(A) Ribbon representation of the a-subunit with the key conserved aR231 and the protonatable cD61 carboxylates in the c10 ring shown in ball-and-stick representation. Periplasmic and cytoplasmic channels are shown in maroon and purple, respectively. Arrow indicates direction of rotation during ATP synthesis. (B) Structurally distinct proton entry route into the periplasmic entry channel of A. baumannii ATP synthase. A structural alignment of A. baumannii (light blue) and Bos taurus (gray) a-subunit is shown in cartoon representation, along with the periplasmic entry channels calculated using HOLLOW (33). Red channel: A. baumannii. Pink channel: B. taurus. The dashed rectangle indicates the zoomed region shown in the right panel figure, with a simplified view of the channels shown in two rotations. Viewing direction is indicated in the left panel and is consistent in the remaining panels (B to H). The equivalent mini-helix 1 residues are colored in magenta and correspond to the region indicated in the a-subunit amino acid alignment (fig. S5A). This helix can assume a proximal (A. baumannii) or distal (B. taurus) position, as indicated. The corresponding proton entry paths from either the front (A. baumannii) or from the rear (B. taurus) are indicated by green and orange arrows. (C to H) Same view as in (B) of the a-subunit, the periplasmic channel, the proton entry route, and the position of mini-helix 1 in various species. (C) A. baumannii, state 1 (7P2Y); (D) B. taurus, 6ZQM (37); (E) M. smegmatis, 7JGA (16); (F) Saccharomyces cerevisiae, 6B8H (38); (G) E. coli, 6OQR (39); and (H) Spinacia oleracea, 6FKF (12). Note that the residues corresponding to mini-helix 1 are not modeled in the M. smegmatis and S. oleracea structures.