Abstract
Background
Heart rate-corrected QT (QTc) interval is associated with increased risk for cardiovascular events and mortality among individuals with diabetes mellitus (DM). Little is known about the epidemiology of prolonged QTc among people with DM in resource-limited settings.
Methods
We conducted a cross-sectional study among adults with diabetes in ambulatory care at the Mbarara Regional Referral Hospital, from November 2018 to April 2019. Twelve-lead ECG recordings were performed on all participants. We collected clinical and laboratory data related to diabetes disease status and treatment control. We estimated QTc using Bazett’s formula and categorized it according to standardized sex-adjusted thresholds. Linear regression analysis was performed to identify correlates of QTc.
Results
We recruited 299 participants with a mean age of 50.1 years (SD±9.8) and mean HbA1c of 9.7 % (SD±2.6), and 69.6% were female. We detected prolonged and borderline QTc in 6.4% (19/299, 95% CI: 3.9–9.7%) and 23.4% (70/299, 95% CI: 18.7–28.6%) of participants, respectively. In multivariate models, factors associated with increasing QTc interval were mean arterial pressure (β=0.34; 95% CI: 0.07–0.63, p=0.019) and female sex (β=15.26; 95% CI: 7.58–22.94, p0.001).
Conclusions
The prevalence of abnormal QTc among individuals in routine diabetes care in southwestern Uganda was high. Female sex and mean arterial pressure were correlated with QTc interval. Given these findings, future studies should explore the clinical impact of abnormal QTc in this patient population.
Keywords: QT interval, Prevalence, Diabetes, QTc dispersion, QTc prolongation, Uganda
Introduction
Morbidity and mortality of diabetes are rising rapidly in low-income countries [1]. In Africa particularly, approximately five percent of deaths are now attributable to diabetes among adults aged 20–60 years [1]. Cardiovascular disease (CVD) in diabetic individuals is often unrecognized, yet it is by far the leading cause of morbidity and mortality in these individuals [2].
A major risk factor for CVD events and mortality in people with diabetes are abnormalities of the cardiac rhythm, including abnormal QT interval. In diabetic individuals, prolonged heart rate-corrected QT (QTc) interval is associated with increased risk of stroke, ischemic heart disease, and all-cause mortality and cardiovascular mortality, including sudden death [3, 4]. Thus, QTc interval on the ECG can potentially be utilized to identify diabetic individuals at high risk for cardiovascular events [5–7].
Whereas the prevalence of long QTc interval among individuals with diabetes varies widely, with higher estimates reported in type 2 diabetes (15 to 67%) [8–10] compared to type 1 diabetes (8%) [11], there are scanty data on its prevalence and correlates in sub-Saharan Africa, where an estimated 25 million people with diabetes reside [1]. We sought to determine the prevalence of QTc interval abnormalities and associated factors among patients with diabetes attending ambulatory care at the Mbarara Regional Referral Hospital (MRRH) in southwestern Uganda, to fill this knowledge gap.
Methods
Study population and setting
Methods for the parent cohort study have already been published previously[12]. Briefly, we conducted a cross-sectional study among patients with diabetes attending the outpatient diabetes and endocrinology clinic of the Mbarara Regional Referral Hospital (MRRH) from November 2018 to April 2019. We included diabetic patients aged 18 to 65 years using consecutive sampling of eligible participants. We defined diabetes in participants with fasting capillary glucose of ≥7.0 mmol/L or those who were already on treatment for diabetes mellitus. We excluded individuals with acute febrile illnesses in the previous 48 h, those with electrolyte abnormalities, and those with other endocrine disorders (e.g., thyroid hormone disorders). We also excluded those who were actively taking drugs known to affect the cardiac rhythm or QTc interval including antiarrhythmic drugs (class I and class III), antihypertensive medications, antidepressants, or digitalis in the prior 24 h. Lastly, we excluded individuals with self-reported or documented history of cardiac (heart failure, ischemic heart disease), renal, or hepatic disease.
Study definitions and procedures
We collected socio-demographic and clinical characteristics, including data on alcohol use, smoking, level of physical activity, and diabetes treatment history of the study participants through a structured questionnaire. We measured weight and height to the nearest 0.1kg and 0.1 cm, respectively, with participants putting on light clothes and removing shoes. We calculated body mass index (BMI) as body weight in kilograms divided by the square of the body height in meters. We measured waist circumference at the level of umbilicus with inelastic tapeline (to the nearest 0.1 cm), at the end of normal expiration.
Blood was collected for glycosylated hemoglobin (HbA1c, Cobas Integra 400, Roche diagnostics, Basel, Switzerland). Fasting blood sugar measurements were obtained using a Freestyle Glucometer (Abbott Diabetes Care Inc., Maidenhead, UK) after at least 8 h of fasting. Blood pressure was recorded in a sitting position using an automatic sphygmomanometer in the upper arm (Omron HEM 705 LP, Omron Healthcare, Inc., Bannockburn, IL, USA). We calculated the pulse pressure as the difference between systolic and diastolic blood pressures in mmHg and computed the mean arterial pressure as the sum of diastolic blood pressure and one-third of pulse pressure. After pupillary dilation by an ophthalmologist, the presence of diabetic retinopathy was diagnosed with direct ophthalmoscopy and categorized as non-proliferative or proliferative diabetic retinopathy.
Measurement and classification of QTc interval
We performed 12 lead ECG recordings on all study participants using a portable ECG machine (Edan Instruments, Inc., Hessen, Germany). Participants were instructed to rest for 5 min in supine position before all the ECG recordings. We measured the QT interval from the beginning of the earliest onset of the QRS complex to the end of the T wave where it crosses the isoelectric line. In case a U wave was present, we measured the QT interval up to the bottom of the angle between the T and U waves. We considered QT as the mean of QT from five consecutive cycles in lead V5. We calculated the QTc interval according to Bazett’s formula (QTc=QT/RR1/2) for heart rates between 60 and 100 beats/minute and Fredericia’s formula (QTc=QT/RR1/3) for heart rates less than 60 or greater than 100 beats/minute because Bazett’s formula tends to over-correct at high heart rates and under-correct at low heart rates [13]. We further classified the QTc interval as normal (<430ms in males, <450ms in females), borderline (430–450ms in males, 450–470ms in females), or prolonged (>450ms in males, >470ms in females) to further adjust for sex, according to a criteria previously described [13]. Finally, we estimated QTc dispersion (QTd) as the difference between the maximum and minimum QTc intervals in V5. QTd of >80ms was considered an abnormally prolonged [9]. To ensure quality control, two independent observers (D.C.A and G.K), who were blinded to participants’ data, measured the QT and RR intervals and an average measure taken. We estimated Pearson’s correlation coefficient to estimate inter-reader agreement between the two observers.
Sample size and statistical analyses
For the parent study, we calculated a sample size of 296 participants to enable a 5% precision with a 95% confidence interval around an estimate of our primary outcome, cardiovascular autonomic neuropathy (CAN), with prevalence of 20%, after consideration of a 10% non-response rate [12]. Statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX, USA).
For this analysis, our outcome of interest was QTc interval. We first categorized the QTc interval into three categories (normal, borderline, and prolonged), according to the following cut-offs: normal (<430ms in males, <450ms in females), borderline (430–450ms in males, 450–470ms in females), and prolonged (>450ms in males, >470ms in females), as per recognized criteria [13]. We determined the prevalence of QTc interval abnormalities as a proportion of participants meeting the definitions of the respective categories. We considered participants to have abnormally long QTc interval if they fell into the category of borderline or prolonged QTc interval. We then described the socio-demographic and clinical characteristics of study participants by the subgrouping of QTc interval and compared differences in socio-demographic and clinical characteristics of those with QTc interval>440ms and those with QTc interval≤440ms, using chi-square test for categorical variables, Student t-tests for continuous normally distributed variables (expressed as means with standard deviations), and Wilcoxon rank-sum test for non-normally distributed continuous variables (expressed as medians with inter-quartile ranges). We performed univariable and multivariable linear regression analyses to determine the factors associated with QTc interval, reporting beta regression coefficients with their 95% confidence intervals as our measures of association. Variables that were considered to have an association (p<0.1) in univariable analyses were entered in the multivariable models through stepwise backward method. The entry and removal probabilities for stepwise were 0.05 and 0.1, respectively. Variables in the multivariable model with p-values <0.05 were considered statistically significant.
Results
We analyzed the data of 299 participants from a total of 512 individuals screened for inclusion into the study. Of the 512 screened individuals, 213 met the exclusion criteria including five with thyroid disorders, 94 who had taken cardiotropic medications (calcium channel blockers, beta blockers) in the prior 24-h period, and 17 with other underlying medical conditions (cardiac, renal, or hepatic disease).
Socio-demographic and clinical characteristics
Participants’ characteristics are summarized in Table 1. Of the 299 participants analyzed, 69.6% were female; mean age was 50.1 years (SD±9.8), mean HbA1c was 9.7% (SD±6.7), and mean duration of diabetes was 5.8 (SD±5.9) years. We found a higher crude prevalence of QTc prolongation among women, those with higher resting systolic and diastolic blood pressures and those with higher pulse pressure and higher mean arterial pressure. Other characteristics were similar between the two groups.
Table 1.
Demographic and clinical characteristics of study participants by QTc interval category
| QTc interval >440ms | QTc interval ≤440ms | ||
|---|---|---|---|
| Characteristic | n = 104 | n = 195 | p value |
| Age in years, mean (±SD) | 51.3 (±9.4) | 49.5 (±10.0) | 0.136 |
| Female sex, n (%) | 87 (83.7) | 121 (62.1) | <0.001 |
| Height in meters, mean (±SD) | 1.62 (0.07) | 1.62 (0.09) | 0.798 |
| Weight in kg, mean (±SD) | |||
| BMI in kg/m2, mean (±SD) | 27.9 (±6.1) | 27.2 (±5.1) | 0.300 |
| Waist circumference in cm, mean (±SD) | 99.0 (±14.0) | 97.8 (±13.4) | 0.460 |
| Ever smoked, n (%) | 26 (25.0) | 43 (22.1) | 0.564 |
| Duration of diabetes in year, median (IQR) | 4 (2.9) | 4 (1.8) | 0.237 |
| Vigorous physical activity (≥600 METS/week) (%) | 66 (63.5) | 107 (54.9) | 0.152 |
| Fasting blood sugar in mmol/L, mean (±SD) | 11.3 (±5.1) | 11.1 (±4.7) | 0.669 |
| HbA1c (%), mean (±SD) | 9.6 (±2.6) | 9.8 (±2.6) | 0.505 |
| History of hypertension, n (%) | 82 (78.9) | 125 (64.1) | 0.009 |
| Resting systolic blood pressure in mmHg, mean (±SD) | 146 (±23) | 140 (±22) | 0.030 |
| Resting diastolic blood pressure in mmHg, mean (±SD) | 89 (±10) | 86 (±11) | 0.023 |
| Mean arterial pressure in mmHg, mean (±SD) | 108 (±13) | 104 (±13) | 0.014 |
| Pulse pressure in mmHg, mean (±SD) | 57 (±18) | 54 (±18) | 0.165 |
| Presence of diabetic retinopathy, n (%) | 0.790 | ||
| None | 81 (77.9) | 150 (76.9) | |
| Non-proliferative | 19 (18.3) | 34 (17.4) | |
| Proliferative | 4 (3.9) | 11 (5.6) | |
| Symptoms of neuropathy in past 6 months n (%) | |||
| Palpitations | 58 (55.8) | 91 (46.7) | 0.134 |
| Fainting | 41 (39.4) | 73 (37.4) | 0.736 |
| Numbness in feet | 67 (64.4) | 116 (59.5) | 0.404 |
| Use of antihypertensive drugs n (%) | 0.246 | ||
| None | 3 (5.5) | 14 (17.5) | |
| Diuretic | 6 (10.9) | 7 (8.8) | |
| Beta blocker | 3 (5.5) | 6 (7.5) | |
| Calcium channel blocker | 44 (32.6) | 21 (26.3) | |
| ACEI/ARB | 17 (30.9) | 25 (31.3) |
SD standard deviation, IQR inter-quartile range, METS metabolic equivalents, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers
Prevalence of QTc interval abnormalities
Pearson’s correlation coefficient for the agreement between the two observers for the QTc intervals was 0.92. We detected abnormally long QTc interval in 89/299 participants (29.8%, 95% CI: 24.6–35.3%), with a prevalence of prolonged QTc of 6.4% (19/299, 95% CI: 3.9–9.7%) and a prevalence of borderline QTc of 23.4% (70/299, 95% CI: 18.7–28.6%). There was no significant difference in the distribution of different categories of QTc interval in males and females, as seen in Fig. 1. No participants had abnormally prolonged QTc dispersion (QTd).
Fig. 1.
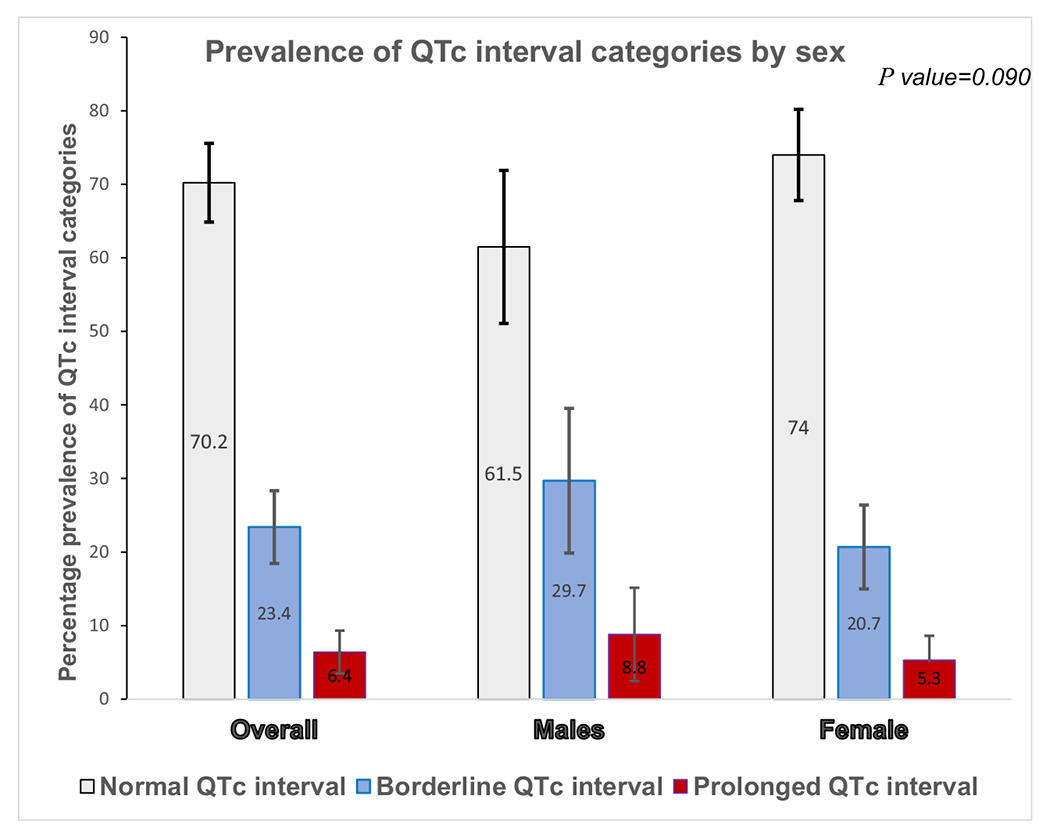
Distribution of QTc interval abnormalities by sex among study participants
Factors associated with QTc interval
Results of univariable and multivariable linear regression models for factors associated with QTc interval are presented in Table 2. In unadjusted analyses, female sex (p<0.001), resting systolic blood pressure (p=0.020), resting diastolic blood pressure (p=0.010), history of hypertension (p=0.007), mean arterial pressure (p<0.001), and history of taking calcium channel blockers (p=0.006) were significantly associated with increasing QTc interval.
Table 2.
Univariate and multivariate linear regression analyses for factors associated with QTc interval
| Unadjusted linear regression analysis |
Adjusted linear regression analysis |
|||
|---|---|---|---|---|
| Variable | β coefficient (95% CI) | p value | β coefficient (95% CI) | p value |
| Age in years | 0.25 (−0.001 to 0.51) | 0.050 | 0.09 (−0.17 to 0.35) | 0.491 |
| Female sex | 15.52 (10.29–20.61) | <0.001 | 15.26 (7.58–22.94) | <0.001 |
| BMI in kg/m2 | 0.31 (−0.14 to 0.76) | 0.182 | ||
| Waist circumference in cm | 0.09 (−0.09 to 027) | 0.334 | ||
| Duration of diabetes in years | 0.38 (−0.04 to 0.80) | 0.074 | 0.18 (−0.75 to 0.39) | 0.532 |
| Fasting blood sugar in mmol/L | −0.06 (−0.57 to 0.46) | 0.832 | ||
| HbA1c (%) | −0.07 (−1.04 to 0.89) | 0.887 | ||
| History of hypertension | 7.39 (2.02–12.76) | 0.007 | ||
| Resting systolic blood pressure in mmHg | 0.18 (0.07–0.29) | 0.002 | ||
| Resting diastolic blood pressure in mmHg | 0.41 (0.18–0.64) | 0.001 | ||
| Mean arterial pressure in mmHg | 0.34 (0.16–0.53) | <0.001 | 0.34 (0.07–0.63) | 0.019 |
| Pulse pressure in mmHg | 0.14 (−0.01 to 0.28) | 0.057 | ||
| Antihypertensive drugs | ||||
| None | Ref | Ref | ||
| Diuretic | 11.72 (−4.25 to 27.69) | 0.149 | 10.07 (−4.97 to 25.11) | 0.187 |
| ACEI/ARB | 11.77 (−0.69 to 24.23) | 0.064 | 10.70 (−1.19 to 22.58) | 0.077 |
| Beta blocker | 8.63 (−9.23 to 26.50) | 0.341 | 4.55 (−13.09 to 22.19) | 0.611 |
| Calcium channel blocker | 17.34 (4.97–29.72) | 0.006 | 14.65 (−0.67 to 26.57) | 0.054 |
CI confidence interval, BMI body mass index, HbA1c glycosylated hemoglobin, ARB angiotensin receptor blocker, ACEI angiotensin-converting enzyme inhibitor, Ref reference category
In the final multivariate model (Table 2), after controlling for the duration of diabetes, age, and antihypertensive medications, significant factors associated with QTc interval were mean arterial pressure (β=0.34; 95% CI: 0.07–0.63, p=0.019) and female sex (β=15.26; 95% CI: 7.58–22.94, p<0.001). The predicted QTc interval for females was higher across all age groups (Fig. 2).
Fig. 2.
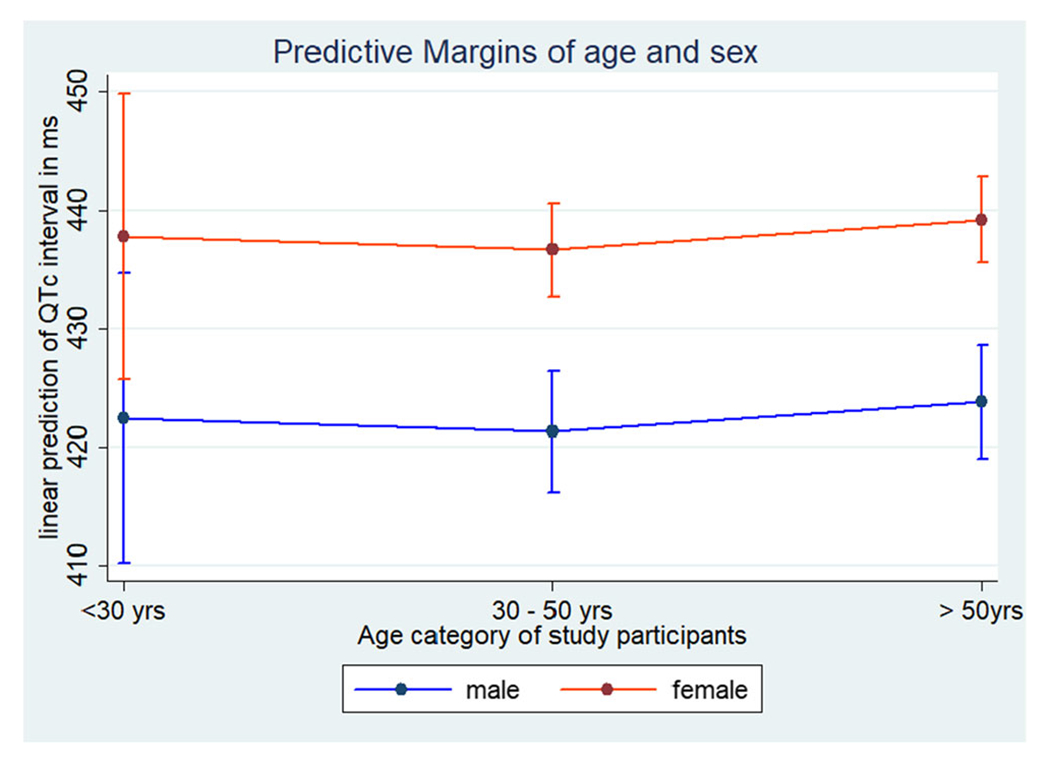
Linear prediction of QTc interval by age and sex with 95% confidence intervals
Discussion
We found a high prevalence of abnormally long QTc interval among individuals with diabetes in ambulatory care in southwestern Uganda and identified female sex and mean arterial pressure as key correlates of increased QTc interval. The prevalence of abnormally long QTc interval of 30% (95% CI: 24.6–35.3%) reported in our study is consistent with studies in Italy and China that have reported prevalence estimates ranging from 24 to 35% among diabetic individuals [9, 10]. In contrast, our study demonstrates much higher prevalence of abnormally long QTc than others from Nigeria and Iraq, which reported much lower estimates of 12% and 9%, respectively, among diabetic individuals [14, 15]. Our study also revealed a significantly higher prevalence (p<0.001) than the 11.7% prevalence recently reported in the general population in southwestern Uganda [16].
These results reinforce previous findings that suggest that individuals with diabetes have increased QTc intervals compared to non-diabetic individuals [9]. This phenomenon is hypothesized to be due to persistent hyperglycemia, which increases intracellular levels of calcium, causes production of free radicals, alters cardiac sympathovagal tone balance, and contributes to diminished nitric oxide production [17]. Low levels of nitric oxide in turn may cause impaired functioning of primary active transport mechanisms within cardiomyocytes, resulting in prolongation of myocardial repolarization [18]. Furthermore, hyperglycemia-mediated free reactive species have been shown to impair functioning of the P-glycoprotein, a protein that is responsible for intracellular extrusion of multiple pro-arrhythmic agents [19] and IKr channels, the main channels that contribute to potassium efflux during repolarization [20]. This combination of factors is believed to alter cardiomyocyte repolarization, ultimately affecting the QTc interval [20].
Our results demonstrated a correlation between mean arterial pressure and QTc interval, in agreement with previous studies [21–24]. Of note, the association between blood pressure and QTc interval has also previously been demonstrated in hypertensive mice [25]. Although the mechanisms by which arterial blood pressure influences QTc interval are not well understood, it is postulated to be due to chronic alterations in the ultrastructure of cardiomyocytes, as a result of chronically increased afterload [26]. Additionally, the predominance of sympathetic nervous system activation, often seen in hypertensive patients, may result into increased QTc interval [27]. The influence of arterial blood pressure on QTc interval demonstrated in our study has clinical implications, as it further confirms the need to better control blood pressure in diabetic patients, so as to reduce the risk of adverse cardiovascular events. Future longitudinal studies will be required to ascertain whether control of blood pressure could potentially result into improvement in QTc interval and cardiovascular events in the study population.
Finally, we corroborated a well-established correlation between QTc interval and sex, with females having a higher QTc interval compared to males, in agreement with previous findings [15, 28]. After puberty, females tend to have a longer baseline QTc interval due to sex-hormone-related differences in modulation of ionic currents within the myocardium [29]. In individuals with diabetes, these sex-related differences in QTc interval duration may be amplified. This finding is in keeping with a possible increased risk for QTc-related cardiovascular events observed in females [30].
Limitations
We cannot demonstrate a causal relationship between measured correlates and QTc interval duration because of the cross-sectional nature of our study design. Our findings are also prone to residual and unmeasured confounding from factors that are known to affect QTc interval, such as serum electrolytes, levels of thyroid hormones, or unreported medications including different antidiabetic medications that may prolong QTc interval. Finally, we conducted a single-center, cross-sectional study without longitudinal outcome data, so we are unable to describe the clinical consequence of traditionally defined QTc prolongation in our study population.
Conclusion
The prevalence of abnormally long QTc interval was high among ambulatory individuals with diabetes in rural Southwestern Uganda, detected in about one-third of the study participants. Female sex and mean arterial pressure were correlated with longer QTc intervals. Our findings give support to consider QTc screening before initiation of QT prolonging agents among diabetics in the region and should lead to additional study of the clinical consequences of prolonged QTc interval in this patient population.
Acknowledgment
We thank the study participants, the staff, and management of the Mbarara Regional Referral Hospital who contributed in several ways towards the success of this study. We are very thankful to members of the Technical Advisory Committee (TAC) of the Mbarara University Research Training Initiative (MURTI) for their technical input into the study. We also acknowledge Dr. Sam Ruvuma and Dr. Kwagga Teddy of the Ophthalmology Department of the Mbarara University of Science and Technology for their technical support.
Funding
This research was supported by the Fogarty International Center and co-founding partners (NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH); Office of AIDS Research, Office of the Director (OAR/NIH); National Institute of Mental Health (NIMH/NIH); and National Institute of Neurological Disorders and Stroke (NINDS/NIH)) of the National Institutes of Health under Award Number D43TW010128.
Footnotes
Ethics approval and consent to participate The study got an approval from the institutional ethics review board of the Mbarara University of Science and Technology (MUST-REC). We also received approval for the study from the Uganda National Council of Science and Technology (UNCST) and from the Research Secretariat in the Office of the President of Uganda, in accordance with the national guidelines. All study participants provided written informed consent before recruitment and participation. Participants who could not write gave consent with a thumbprint.
Conflict of interests The authors declare no competing interests.
Publisher's Disclaimer: Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
The datasets generated and analyzed during the study are available from the corresponding author on request.
References
- 1.Organization, W.H. Global report on diabetes: World Health Organization; 2016. [Google Scholar]
- 2.Schernthaner G, Lotan C, Baltadzhieva-Trendafilova E, Ceponis J, Clodi M, Ducena K, et al. Unrecognised cardiovascular disease in type 2 diabetes: is it time to act earlier? Cardiovasc Diabetol. 2018;17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso CR, Salles GF, Deccache W. QTc interval prolongation is a predictor of future strokes in patients with type 2 diabetes mellitus. Stroke. 2003;34(9):2187–94. [DOI] [PubMed] [Google Scholar]
- 4.Veglio M, Sivieri R, Chinaglia A, Scaglione L, Cavallo-Perin P. QT interval prolongation and mortality in type 1 diabetic patients: a 5-year cohort prospective study. Neuropathy Study Group of the Italian Society of the Study of Diabetes, Piemonte Affiliate. Diabetes Care. 2000;23(9):1381–3. [DOI] [PubMed] [Google Scholar]
- 5.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV, Strong Heart Study. Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the strong heart study. Diabetes. 2004;53(2):434–40. [DOI] [PubMed] [Google Scholar]
- 6.Rossing P, Breum L, Major-Pedersen A, Sato A, Winding H, Pietersen A, et al. Prolonged QTc interval predicts mortality in patients with type 1 diabetes mellitus. Diabet Med. 2001;18(3):199–205. [DOI] [PubMed] [Google Scholar]
- 7.Salles GF, Bloch KV, Cardoso CR. Mortality and predictors of mortality in a cohort of Brazilian type 2 diabetic patients. Diabetes Care. 2004;27(6):1299–305. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Ren H, Xu ZR, Liu YJ, Yang XP, Liu JQ. Prevalence and risk factors of prolonged QTc interval among Chinese patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veglio M, et al. Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Intern Med. 2002;251(4):317–24. [DOI] [PubMed] [Google Scholar]
- 10.Yang X-h, et al. The relationship between insulin sensitivity and heart rate–corrected QT interval in patients with type 2 diabetes. Diabetol Metab Syndr. 2017;9(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivieri R, Veglio M, Chinaglia A, Scaglione P, Cavallo-Perin P, For the Neuropathy Study Group of the Italian Society for the Study of Diabetes, Piemonte affiliate. Prevalence of QT prolongation in a type 1 diabetic population and its association with autonomic neuropathy. Diabet Med. 1993;10(10):920–4. [DOI] [PubMed] [Google Scholar]
- 12.Migisha R, et al. Prevalence and correlates of cardiovascular autonomic neuropathy among patients with diabetes in Uganda: a hospital-based cross-sectional study. Glob Heart. 2020;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17(3):333–6. [DOI] [PubMed] [Google Scholar]
- 14.Ukpabi OJ, Onwubere BJ. QTc prolongation in Black diabetic subjects with cardiac autonomic neuropathy. Afr Health Sci. 2017;17(4):1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahwi TO, Hasan RM, Faraj HI. Incidence of QTc prolongation in type 2 diabetes mellitus and its relation to cardiac autonomic neuropathy. J Med J. 2014;48(2):102–11. [Google Scholar]
- 16.Magodoro IM, et al. Population prevalence and correlates of prolonged QT interval: cross-sectional, population-based study from rural Uganda. Glob Heart. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Phys Heart Circ Phys. 1992;263(2):H321–6. [DOI] [PubMed] [Google Scholar]
- 18.Fiorentini A, Perciaccante A, Valente R, Paris A, Serra P, Tubani L. The correlation among QTc interval, hyperglycaemia and the impaired autonomic activity. Auton Neurosci. 2010;154(1-2):94–8. [DOI] [PubMed] [Google Scholar]
- 19.Wartenberg M, Ling FC, Schallenberg M, Bäumer AT, Petrat K, Hescheler J, et al. Down-regulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by reactive oxygen species. J Biol Chem. 2001;276(20):17420–8. [DOI] [PubMed] [Google Scholar]
- 20.Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. 2005. Am Heart Assoc. [DOI] [PubMed] [Google Scholar]
- 21.Satpathy S, Satpathy S, Nayak PK. Correlation of blood pressure and QT interval. Natl J Physiol Pharm Pharmacol. 2018;8(2):207–10. [Google Scholar]
- 22.Sun G-Z, Zhou Y, Ye N, Wu SJ, Sun YX. Independent influence of blood pressure on QTc interval: results from a general Chinese population. Biomed Res Int. 2019;2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng S, Yu Y, Hao K, Xing H, Li D, Chen C, et al. Heart rate-corrected QT interval duration is significantly associated with blood pressure in Chinese hypertensives. J Electrocardiol. 2006;39(2):206–10. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura K, et al. Association of QT interval with blood pressure in 80-year-old subjects. Hypertens Res. 2004;27(6):387–91. [DOI] [PubMed] [Google Scholar]
- 25.Klimas J, Stankovicova T, Kyselovic J, Bacharova L. Prolonged QT interval is associated with blood pressure rather than left ventricular mass in spontaneously hypertensive rats. Clin Exp Hypertens. 2008;30(7):475–85. [DOI] [PubMed] [Google Scholar]
- 26.Oikarinen L, Nieminen MS, Toivonen L, Viitasalo M, Wachtell K, Papademetriou V, et al. Relation of QT interval and QT dispersion to regression of echocardiographic and electrocardiographic left ventricular hypertrophy in hypertensive patients: the Losartan Intervention for Endpoint Reduction (LIFE) study. Am Heart J. 2003;145(5):919–25. [DOI] [PubMed] [Google Scholar]
- 27.Marfella R, Gualdiero P, Siniscalchi M, Carusone C, Verza M, Marzano S, et al. Morning blood pressure peak, QT intervals, and sympathetic activity in hypertensive patients. Hypertension. 2003;41(2):237–43. [DOI] [PubMed] [Google Scholar]
- 28.Giunti S, Bruno G, Lillaz E, Gruden G, Lolli V, Chaturvedi N, et al. Incidence and risk factors of prolonged QTc interval in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care. 2007;30(8):2057–63. [DOI] [PubMed] [Google Scholar]
- 29.Griffin M, Lee HW, Zhao L, Eghbali-Webb M. Gender-related differences in proliferative response of cardiac fibroblasts to hypoxia. Mol Cell Biochem. 2000;215(1-2):21–30. [DOI] [PubMed] [Google Scholar]
- 30.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164(9):943–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the study are available from the corresponding author on request.


