Abstract
Objectives:
To present consensus statements and supporting literature for plasma and platelet transfusions in critically ill children with severe trauma, traumatic brain injury (TBI) and/or intracranial hemorrhage (ICH) from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB).
Design:
Systematic review and consensus conference of international, multidisciplinary experts in platelet and plasma transfusion management of critically ill children.
Setting:
Not applicable.
Patients:
Critically ill neonates and children with severe trauma, traumatic brain injury (TBI) and/or intracranial hemorrhage (ICH).
Interventions:
None
Measurements and Main Results:
A panel of 8 experts developed expert based statements for plasma and platelet transfusions in critically ill neonates and children with severe trauma, TBI and/or ICH. These statements were reviewed and ratified by the 29 TAXI-CAB experts. A systematic review was conducted using MEDLINE, EMBASE, and Cochrane Library databases, from inception to December 2020. Consensus was obtained using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Results were summarized using the Grading of Recommendations Assessment, Development, and Evaluation method. We developed 1 good practice statement and 6 expert consensus statements.
Conclusions:
The lack of evidence precludes proposing recommendations on monitoring of the coagulation system and on plasma and platelets transfusion in critically ill pediatric patients with severe trauma, severe TBI or non-traumatic ICH.
Keywords: child, critical care, evidence-based, guidelines, intensive care, intracranial hemorrhage, pediatrics, plasma, platelet, transfusion, trauma, traumatic brain injury
INTRODUCTION
Plasma transfusions are frequently prescribed to treat or to prevent bleeding caused by a coagulation disorder. Likewise, platelets are transfused to treat or to prevent bleeding caused by thrombocytopenia or platelet dysfunction. Among 356,583 pediatric trauma patients recorded in the American College of Surgeons National Trauma Databank (2010–2012), 1072 (7.6%) received at least one plasma transfusion, 886 (6.3%) received at least one platelet transfusion, 96 (0.7%) received whole blood, and 173 (0.04%) received a massive transfusion (MT) [1].
In 2013, the Joint Commission and the American Medical Association Convened Physician Consortium National Summit ranked transfusion of blood products second among five overused treatments that may affect patient safety [1, 2]. The transfusion of plasma and platelets can be life-saving, but may also cause clinically significant adverse effects, such as acute respiratory distress syndrome and circulatory overload [3]. In national audits conducted in the United Kingdom, about 20% of blood product usage is outside guideline recommendations [4]. Clearly, clinical decision-making can be improved to best guide plasma and platelet transfusion practice in patients admitted to pediatric intensive care units (PICU). This sentiment is also true for critically ill pediatric patients with severe trauma, moderate to severe traumatic brain injury (TBI) or non-traumatic intracranial hemorrhage (ICH).
There is some evidence that protocols or guidelines can improve clinical practice in the ICU environment [5–12]. The purpose of “Transfusion and Anemia Expertise Initiative - Control/Avoidance of Bleeding” (TAXI-CAB) was to use structured and systematic methods to help practitioners in their decision-making when considering plasma and platelet transfusion in critically ill children. In this paper, we report our assessment of the literature on plasma and platelet transfusion in pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH admitted to the pediatric intensive care unit (PICU), i.e., by definition experiencing critical illness.
METHODS
The search strategy, item selection and recommendation generation used to identify and select references for systematic review and to develop recommendation are detailed in the general manuscript of TAXI-CAB [13]. Briefly, we searched Ovid MEDLINE®, Ovid EMBASE, and Cochrane Library (Wiley) from inception through December 2020 using a combination of medical subject heading terms and text words to define concepts of plasma or platelet transfusion, transfusion triggers, laboratory tests to assess efficacy of transfusion in children admitted to the PICU with severe trauma, severe TBI and/or ICH. For articles selected for inclusion, reference lists and citing articles were selected from Scopus (Elsevier) and screened. Two reviewers independently reviewed all citations and performed data extraction and assessments of bias. Literature was reviewed for relevance to this subgroup. Research Electronic Data Capture (REDCap) hosted at Weill Cornell Medicine was used for standardized data extraction. We used a standardized data extraction form to construct evidence tables and graded the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [14].
Eight experts participated in the development of recommendations from this subgroup (five for severe trauma and three for TBI and/or ICH). A panel of 29 experts convened in an on-line format over 18 months to develop good practice statements, recommendations and, when evidence was lacking, expert consensus statements. Good practice statements are those in which there is high-level of certainty that the practice will do more good than harm, but there is little in the way of supporting literature evidence. Expert consensus statements are based on the expert opinion of the group, but in areas where research is likely needed. All statements from each subgroup were reviewed by the full panel of experts and voted on using the Research and Development/University of California, Los Angeles (UCLA) Appropriateness Method. Agreement was defined a priori as >80% of all experts. The recommendations and statements are intended to apply to infants, children and adolescents. Prophylactic transfusions are those prescribed to patients at risk of bleeding, whereas therapeutic transfusions are given to those with active bleeding.
Definitions
Severe trauma –
Acute body injury caused by any kind of physical accidental and non-accidental trauma, including blunt and penetrating trauma (e.g., significant hemorrhagic trauma to the torso). In this study, we did not consider severe burn as traumatic cases.
Traumatic brain injury (TBI) –
Acute brain injury caused by any kind of physical accidental and non-accidental trauma, including blunt and penetrating trauma. We did not consider as TBI cases of hypoxic brain injury caused by near-drowning, strangulation or acute respiratory insufficiency, nor cases of intoxication.
Intracranial hemorrhage (ICH) –
Acute intracranial venous or arterial hematoma or intracerebral bleeding caused by a traumatic injury or by the rupture of an intracranial blood vessel.
Deteriorating neurologic function –
Was considered to be present if the patient had clinical deterioration in examination with worsening neurologic deficit, decreasing Glasgow coma scale (GCS) score, or change in pupillary light reflex. This finding could be caused by elevated intracranial pressure over 20 mmHg or expanding cerebral hematoma.
Bleeding –
Was defined as severe or moderate according to the Bleeding Assessment Scale in Critically Ill Children (BASIC) [15].
Hemorrhagic shock –
Defined as hemodynamic instability caused by a severe bleeding.
RESULTS
Study selection
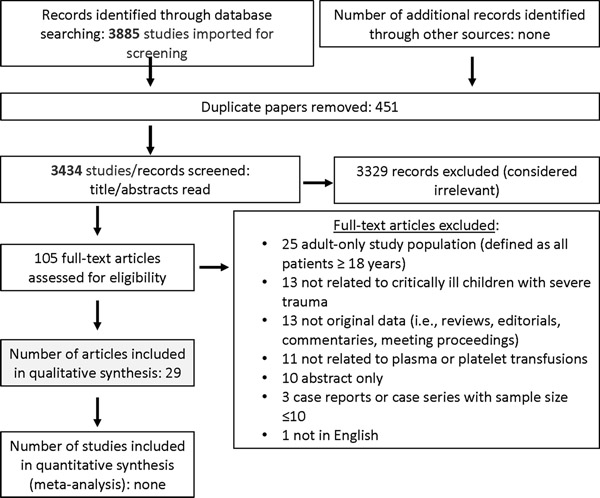
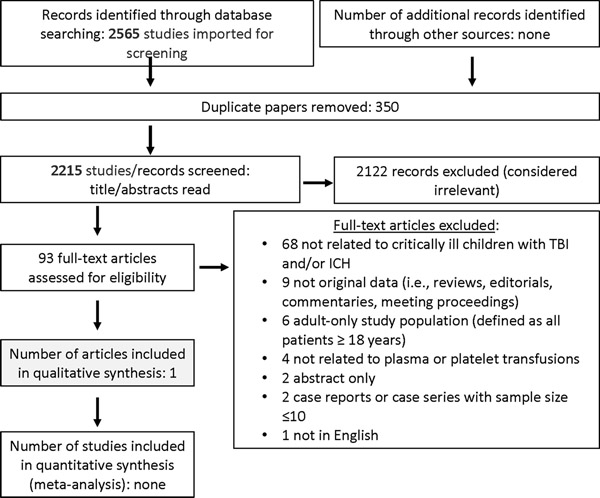
Searching studies for (1) severe trauma and (2) TBI and/or ICH identified 3885 and 2565 abstracts, respectively. After duplicates were removed, a total of 3434 and 2215 were screened. Then, out of 105 and 93 full text manuscripts about (1) severe trauma and (2) TBI and/or ICH, respectively, we selected 29 and 1 paper for detailed review (see Figures 1 and 2). These papers underwent data extraction and assessment of bias in order to generate statements (see Supplemental Data Tables 1 and 2); one good practice statement and six expert consensus statements were produced. The voting data, including the number of voting experts and median score, are provided for each statement.
Figure 1: Papers flow chart for critically ill children with severe trauma.
PRISMA diagram for studies on plasma and/or platelets in critically ill children with severe pediatric trauma.
Figure 2: Papers flow chart for critically ill children with TBI and ICH.
PRISMA diagram for studies on plasma and/or platelets in critically ill children with traumatic brain injury and/or intracranial hemorrhage.
Good practice statement
-
1
In critically ill pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH, we suggest close monitoring of bleeding, including evaluation of potential expansion of ICH, and serial evaluation of the coagulation system. 83% Agreement (n=24), Median 8 (IQR 7–9).
Rationale –
Clinically significant coagulopathy is frequent in children with severe trauma. It can cause major hemorrhage and/or hemorrhagic shock. Severe TBI by itself can cause a clinically significant coagulation disorder [16]. Miner et al. [17] reported that 71% of children with severe head trauma show some alteration of the coagulation system (platelets, prothrombin time, fibrinogen, etc.), including evidence of disseminated intravascular coagulopathy (DIC) in some cases. While, the pathophysiology of trauma-induced coagulopathy is debated, an imbalance between procoagulant factors, anticoagulant factors, platelets, endothelium and fibrinolysis is present [18]. Additionally, hypothermia, acidosis and resuscitation with hypocoagulable fluids (i.e., hemodilution) can worsen trauma-induced coagulopathy.
There is an absence of high-quality data to guide best plasma and platelet transfusion strategies in pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH. Nevertheless, given the available data, we suggest serial evaluation of the coagulation system, including standard coagulation tests - prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), fibrinogen and/or platelet count - in pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH. In patients who are having active resuscitation for hemorrhagic shock, serial monitoring of the blood volume and coagulation system is suggested while active resuscitation is ongoing. The frequency of the monitoring is at the discretion of the provider. Once bleeding stops (either by medical or surgical interventions), liberalization of the monitoring can be considered until values trend toward normal. In some instances, viscoelastic testing (VET) (thromboelastometry, TEG, or rotational thromboelastometry, ROTEM) can be considered to help decision-making (see statement #4).
Expert consensus statements
-
2
In critically pediatric patients with severe trauma, moderate to severe traumatic TBI or in critically ill children with non-traumatic ICH, there is insufficient evidence to make any recommendation regarding specific indications or transfusion strategies to direct plasma or platelet transfusion. 83% Agreement (n=24), Median 8 (IQR 7–9).
Rationale –
There is insufficient strong high-quality evidence needed in order to make any recommendations regarding indications for plasma or platelet transfusion in pediatric patients with severe trauma, severe traumatic ICH or in critically ill children with non-traumatic ICH.
-
3
In critically injured pediatric patients in hemorrhagic shock following trauma, a resuscitation strategy of red blood cells (RBCs), plasma, and platelets in ratios between 2:1:1 to 1:1:1 might be considered. 90% Agreement (n=21), Median 8 (IQR 7.5–9).
Rationale –
MT may be required in critically injured pediatric patients in hemorrhagic shock following trauma. While there are many definitions of MT, each differ in timeframe and volume of blood products administered. For example, giving >10% of blood volume per minute, >50% within 3 hours, >100% of total blood volume or >10 RBC units in adults within 24 hours are considered as MT by different investigators [19]. Neff et al. [20] defined MT as the transfusion of ≥ 40 ml/kg of blood products over 24 hours because this volume/day reliably identifies critically injured children at high risk for early in-hospital death. The incidence rate of MT, when defined as 40 mL/kg/24 h, among 356,583 pediatric civilian trauma patients was 0.04% [1]. MT is rarely required in pediatric trauma, but it is potentially life-saving in patients with hemorrhagic shock.
MT can be delivered by the transfusion of RBC, plasma and platelets altogether in a given ratio or with transfusion of whole blood. Kinslow et al [19] conducted a systematic review on the efficacy of different plasma/RBC ratios (note the sequence: not RBC/plasma ratios as listed in the statement) to decrease mortality in pediatric trauma patients who require a MT [21–26]. The definition of MT differed between all these studies. Three reported a significant improvement in 24-hour mortality with plasma/RBC ratio ≥ 1:1 [22, 23] or ≥ 1:2 [21]. Another study [27] reported no benefit with high plasma/RBC ratio in 364 combat-injured pediatric trauma patients who received a MT. Inaba et al. [28] also reported no improvement in survival with plasma transfusion (17.3% vs. 14.1%; p = 0.30) irrespective of plasma/RBC ratio. On the other hand, the risk of deep venous thrombosis was increased in severely injured children who received plasma/RBC and platelets/RBC ratio ≥ 2:1 [21]. The outcomes of these studies are inconsistent (3 positive, 5 negative), and it is difficult to ascertain specific effects of plasma or platelets as they are often given in tandem in an effort toward balanced resuscitation. No pediatric randomized controlled trial (RCT) demonstrated survival benefit with high or low ratios. Despite the lack of strong pediatric evidence, early replacement therapy with ratios between 1:1:1 or 2:1:1 for RBC/plasma/platelets is advocated by experts in pediatric intensive care medicine [29–31].
The Traumatic Hemostasis and Oxygenation Research (THOR) network suggests using whole blood rather than blood components to treat some cases of severe acute hemorrhage [31–33]. One clinical study supports the use of whole blood in pediatric trauma [33].
Even though there is a lack of evidence in pediatric patients, the TAXI-CAB experts concluded that a balanced resuscitation strategy/ratio for RBC/plasma/platelet of 1:1:1 or 2:1:1 in injured children with hemorrhagic shock or with life-threatening hemorrhage might be considered. This transfusion strategy can be stopped once the hemorrhage is controlled.
-
4
In critically ill pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH, viscoelastic testing (e.g., TEG, ROTEM) might be considered as an adjunct to standard laboratory hemostatic testing to inform decisions regarding the transfusion of plasma and/or platelets. 95% Agreement (n=22), Median 8.5 (IQR 7.75–9).
Rationale –
Within minutes of injury, a high percentage of severely injured patients with hemorrhagic shock develop a clotting abnormality termed Trauma Induced Coagulopathy (TIC). TIC was observed in 57% of 956 patients younger than 17 years of age with severe trauma [34]. In this study, coagulopathic children received more RBC and plasma transfusions and had fewer ICU (p=0.042) and ventilator-free days (p=0.007) and higher mortality (p <0.001); the difference in mortality was also seen in those patients with severe TBI (p=0.002) [26]. Abnormal TEG, ROTEM, PT, aPTT and/or low platelet count are associated with a higher risk of mortality [35–38]. Leeper et al. [39] reported an association between a higher INR and the risk of mortality in 776 pediatric trauma patients. Deng et al. [40] reported that a ROTEM-guided protocol improves the outcomes of pediatric trauma patients. Thus, close monitoring of the coagulation system might be considered in critically ill pediatric patients with severe trauma, moderate to severe TBI, and may also be beneficial in children with ICH.
However, the best and most appropriate testing strategy is still a matter of debate. Standard tests such as PT/INR, aPTT, platelet count and fibrinogen might be considered. Two systematic reviews suggest that using TEG or ROTEM to help with decision-making about transfusion strategy reduces the amount of bleeding in adults with MT [41, 42]. However, the pediatric data embedded in these systematic reviews were collected in two trials recruiting 131 cases of elective cardiac surgery with cardiopulmonary bypass, not in PICU patients with severe trauma, TBI and/or ICH [43, 44]. The best evidence that we have on which coagulation tests may be considered in trauma patients comes from the “Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy (iTacTic)” RCT [45]. This large pragmatic trial aimed to determine whether augmenting major hemorrhage protocols (MHPs) with either point-of-care VET would improve outcomes compared to Conventional Coagulation Tests (CCTs). The RCT included 396 adults with trauma-related major hemorrhage activating a MT protocol. At 24 hours, there was no difference in the proportion of patients with the composite primary outcome of participants who were alive and free of MT (VET: 67%, CCT: 64%, Odds Ratio (OR) 1.15, 95% CI 0.76–1.73). Moreover, there was no difference in 28-day mortality (VET: 25%, CCT: 28%, OR 0.84, 95% CI 0.54–1.31), or in other secondary outcomes or serious adverse events. In the pre-specified subgroup of 74 patients with TBI, 64% were alive and free of MT at 24 h compared to 46% in the CCT arm (OR 2.12, 95% CI 0.84–5.34). The main conclusion of iTacTic was: “In adult trauma patients presenting with signs of haemorrhagic shock, [there was] no benefit of viscoelastic haemostatic assay augmented protocols when compared to conventional coagulation tests augmented protocols.”
What are the data specific to pediatric trauma patients? Leeper et al [46] concluded that plasma transfusion should not be targeted to INR thresholds but rather to TEG activated clotting time and clinical bleeding; however, this conclusion is not supported by data. Rowell et al [47] also concluded that routine plasma transfusion to correct a moderately elevated INR before neurological intervention should be re-examined. Bauer et al. [48] reported that the transfusion of plasma in children with acute TBI based on TEG rather than INR may lead to improved outcomes. Some pediatric intensivists state that coagulation tests including abnormal INR and VET could prompt them to prescribe plasma in patients assessed to be at high risk for bleeding when adequate surgical hemostasis is achieved, even in non-bleeding patient [49].
In summary, there are no convincing data on which coagulation system test should be utilized to monitor patients with severe trauma, moderate to severe TBI or non-traumatic ICH. Standard laboratory hemostatic testing might be considered in all pediatric patients with VETs used as adjuncts to inform decisions regarding the transfusion of plasma and/or platelets in these populations.
-
5
In a neurologically stable critically ill pediatric patient with severe trauma, moderate or severe TBI and/or ICH, platelet transfusion if the platelet count is > 100×109/L (100,000/mm3) may not be beneficial. 91% Agreement (n=23), Median 9 (IQR 8–9).
Rationale –
The consensus that platelet transfusion may not be beneficial in neurologically stable critically ill pediatric patient with severe trauma, moderate or severe TBI and/or ICH if the platelet count is > 100 × 109/L was strong (91% agreement).
The American Society of Anesthesiologists considers that a platelet transfusion might be indicated despite an adequate platelet count if a platelet dysfunction is known or suspected, including if the patient is receiving an antiplatelet agent [50].
In contrast to the above statement, in situations with neurologic instability, i.e. the GCS is deteriorating, increasing intracranial pressure (ICP), decreasing cerebral perfusion pressure or increasing seizure activity, it is unclear if the transfusion of platelets may be beneficial even when the platelet count is > 100×109/L if one suspects that the neurological deterioration could be attributable to expanding intracranial hematoma or intracerebral hemorrhage and other causes are ruled out.
Consensus was not reached on a threshold platelet count that could guide platelet transfusion if the platelet count is ≤ 100×109/L. The American Society of Anesthesiologists suggests a threshold of 50×109/L in surgical patients in the presence of significant bleeding [50].
-
6
If an ICP monitoring device must be inserted in a neurologically deteriorating critically ill pediatric patient with TBI and/or ICH, platelet transfusion might be considered if the platelet count is < 100×109/L (100,000/mm3). 86% Agreement (n=22), Median 7.5 (IQR 7–9).
Rationale –
In the absence of published studies, we asked the following question to members of TAXI-CAB: “Do you think we should set platelet count thresholds for placement of intracranial pressure (ICP) monitors in critically ill children with TBI and/or ICH?”. Twenty-one responses were received, 12 positives (57%), 9 negatives (43%). We also asked: “If you think we should set thresholds, below what platelet count would you suggest that platelets be transfused prophylactically prior to ICP monitor insertion?” We received 13 responses: 6 respondents choose a threshold platelet count of 100×109/L and 3 choose a threshold of 50×109/L. Thereafter, the statement above received strong agreement (86%). The American Society of Neurosurgery, the American Society of Anesthesiology and the C17 Council [50, 51] make no recommendations for a threshold platelet count levels for placement of ICP monitor.
-
7
If an ICP monitoring device must be inserted in a neurologically deteriorating critically ill pediatric patient with TBI and/or ICH, plasma transfusion if the INR is ≤ 1.5 may not be beneficial. 87% Agreement (n=23), Median 8 (IQR 8–9).
Rationale –
For neurosurgery and spine guidelines, professional organizations have no recommendations on INR for placement of ICP monitor. However, in practice, INR is frequently used to support a decision to give a plasma transfusion before the placement of an ICP monitor. When also we asked 21 members of TAXI-CAB if they think we should set INR thresholds for placement of ICP monitors in critically ill children with TBI and/or ICH, eleven respondents believe that we should not. Bauer et al. [48] reported no hemorrhage after the placement of ventriculostomy in 31 TBI patients with INR ≤ 1.6. Davis et al. [52] stated that plasma transfusion to prevent bleeding after placement of ICP monitors results in no change when INR < 1.7 and may delay monitor placement. In a point-prevalence study on 443 patients who received at least one plasma transfusion in 101 PICU in 21 countries, Karam et al [53] reported plasma transfusion significantly improved INR only if the baseline INR was > 2.5. Given these data, we propose that giving a plasma transfusion if the INR is ≤ 1.5 may not be beneficial.
We did not reach consensus regarding management when the INR is > 1.5. However, there is some evidence that plasma transfusion significantly improved INR only in patients with a baseline INR greater than 2.5 (66). In addition, we do not know if there should be different thresholds between extra-axial versus intra-ventricular drain.
DISCUSSION
In practice, there is a consensus that monitoring of the coagulation system is suggested in children with severe trauma and TBI, given the high frequency of coagulopathy in these populations. The correct practice is less obvious in patients with ICH. Which test(s) should be obtained and at what frequency is unclear.
Plasma is transfused to correct multiple coagulation factor deficiencies. It should be transfused in critically ill children with severe trauma, TBI and/or ICH to treat active hemorrhage attributable to a coagulation abnormality or to prevent bleeding prior to an invasive procedure in a patient with abnormal hemostasis. The lack of evidence precludes any recommendation about plasma transfusion, other than that plasma transfusion may not be beneficial if the INR is ≤ 1.5, even when an ICP monitoring device must be inserted. In practice, clinical judgment is the best key to guide plasma transfusion in these populations.
Platelets are transfused to increase the platelet count in patients with thrombocytopenia and/or clinically significant platelet dysfunction. They are transfused in critically ill children with severe trauma, TBI and/or ICH to treat active hemorrhage attributable to thrombocytopenia or platelet dysfunction or to prevent bleeding prior to an invasive procedure with increased risk of bleeding. The lack of evidence precludes any recommendation about platelet transfusion.
It is possible that the risk of bleeding with thrombocytopenia is higher in children than in adults. In a pre-specified post-hoc analysis of the Prophylactic Platelet Dose on Transfusion Outcomes (PLADO) trial, the risk of bleeding was higher with similar platelet count in children than in adults given prophylactic platelet transfusions for treatment-induced hypoproliferative thrombocytopenia (post-hoc analysis) (67). This does not establish specific platelet count thresholds to guide the transfusion practice, but it suggests that we must be more cautious with children.
CONCLUSIONS
There is insufficient pediatric evidence to support specific thresholds for coagulation tests, including INR, VET and platelet count, and the transfusion of plasma and platelets in critically ill pediatric patients with severe trauma, moderate to severe TBI or non-traumatic ICH. The TAXI-CAB good practice and expert consensus statements put forward in this paper are derived from data collected in adults and/or from guidelines for adults.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all members of TAXI-CAB for their support and input, especially during the COVID-19 pandemic. In addition, we thank the Chaire Héma-Québec-Bayer en médecine transfusionnelle de l’Université de Montréal, the American Association of Neurological Surgeons, the Society for the Advancement of Blood Management, the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, the International Society of Blood Transfusion, the Society for Critical Care Medicine, and the AABB for their support.
Financial Support: The Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute under award number R13 HL154544-01.
Copyright Form Disclosure: Drs. Russell and Nellis received support for article research from the National Institutes of Health. Dr. Haas received funding from Octapharma. Dr. Nishijima received funding from BMS. The remaining authors have disclosed that they do not have any potential conflicts of interest.
APPENDIX 1. Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members
(* for executive committee) Co-chairs: Marianne E. Nellis, MD, MS*, Weill Cornell Medicine, New York, NY, and Robert I. Parker, MD*, Renaissance School of Medicine, State University of New York at Stony Brook, Stony Brook, NY; Content Experts: Section 1. Laboratory assays used to assess need for plasma and/or platelet transfusions: Scot T. Bateman, MD*, University of Massachusetts Medical School, Worcester, MA, Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Kenneth E. Remy, MD, MHSc, MSCI, Washington University of St. Louis, St. Louis, MO, Katherine Steffen, MD, Stanford University, Palo Alto, CA; Section 2. Traumatic brain injury and intracranial hemorrhage: David F. Bauer, MD, MPH, Baylor College of Medicine, Houston, TX, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA; Section 3. Following cardiopulmonary bypass: Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Sitaram Emani, MD, Harvard Medical School, Boston, MA, Juan Ibla, MD, Harvard Medical School, Boston, MA, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 4. Supported by extracorporeal membrane oxygenation: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Jill M. Cholette, MD, University of Rochester Golisano Children’s Hospital, Rochester, NY, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 5. Following severe trauma: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Daniel Nishijima, MD, Davis School of Medicine, Davis, CA, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 6. With oncologic diagnosis or following hematopoietic stem cell transplantation: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Lani Lieberman, MD, University of Toronto, Canada, Simon J. Stanworth, MD, University of Oxford, UK, Marie E. Steiner, MD, MS, University of Minnesota, Minneapolis, MN; Section 7. With acute liver failure or following liver transplantation: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 8. Following non-cardiac surgery: Susan M. Goobie, MD, Harvard Medical School, Boston, MA, Thorsten Haas, MD, University Children’s Hospital Zurich, Switzerland, Marisa Tucci, MD, Université de Montréal, Montreal, QC, Canada, Adam M. Vogel, MD, Baylor College of Medicine, Houston, TX; Section 9. Invasive procedures outside of the operating room: Gemma Crighton, MD, Royal Children’s Hospital, Melbourne, Australia, Jacques Lacroix, MD, Université de Montréal, Montreal, QC, Canada, Robert T. Russell, MD, MPH, University of Alabama Birmingham, Birmingham, AL, Paul A. Stricker, MD, Perelman School of Medicine at the University of Pennsylvania, PA; Section 10. Sepsis and/or disseminated intravascular coagulation: Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Simon J. Stanworth, MD, University of Oxford, UK, Katherine Steffen, MD, Stanford University, Palo Alto, CA, Stacey L. Valentine, MD, MPH*, University of Massachusetts Medical School, Worcester, MA; Section 11. Product processing and selection: Meghan Delaney, DO, MPH, The George Washington University Health Sciences, Washington, DC, Ruchika Goel, MD, MPH, Johns Hopkins University, Baltimore, MD, Oliver Karam, MD, PhD*, Children’s Hospital of Richmond at VCU, Richmond, VA, Jennifer A. Muszynski, MD, MPH, Nationwide Children’s Hospital, Columbus, OH; Evidence-based medicine: Melania M. Bembea, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD, Diana Delgado and Michelle Demetres, Weill Cornell Medicine, New York, NY; Implementation science: Katherine Steffen, MD, Stanford University, Palo Alto, CA.
Footnotes
Conflicts: Dr Thorsten Haas is consultant for Octapharma. Dr Daniel Nishijima is consultant for Bristol-Myers-Squibb. The other authors did not report any conflicts of interest.
Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB) Members are listed in Appendix 1
Social media tweet
#Bleeding caused by #coagulopathy or low #platelet count should guide plasma and platelet #transfusion in severe pediatric trauma, brain injury or intracranial hemorrhage.
REFERENCES
- 1.Shroyer MC, Griffin RL, Mortellaro VE, et al. Massive transfusion in pediatric trauma: analysis of the National Trauma Databank. J Surg Res 2017;208:166–172. [DOI] [PubMed] [Google Scholar]
- 2.The Joint Commission and the American Medical Association-Convened Physician Consortium. National Summit on Overuse. In: Physician Consortium for Performance Improvement® (PCPI®), editor. Proceedings from the National Summit on Overuse 2013. p. http://www.jointcommission.org/overuse_summit/
- 3.Bolton-Maggs PHB, on behalf of the SHOT Steering Group: The 2015 Annual SHOT Report. London: Serious Hazards of Transfusion; 2016 [Google Scholar]
- 4.Hibbs SP, Nielsen ND, Brunskill S, et al. The impact of electronic decision support on transfusion practice: a systematic review. Transfus Med Rev 2015;29:14–23. [DOI] [PubMed] [Google Scholar]
- 5.Sinuff T, Muscedere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 2013;41:2627–2640. [DOI] [PubMed] [Google Scholar]
- 6.Baer VL, Henry E, Lambert DK, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion 2011;51:264–269. [DOI] [PubMed] [Google Scholar]
- 7.Stricker PA, Fiadjoe JE, Kilbaugh TJ, et al. Effect of transfusion guidelines on postoperative transfusion in children undergoing craniofacial reconstruction surgery. Pediatr Crit Care Med 2012;13:e357–362. [DOI] [PubMed] [Google Scholar]
- 8.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 9.Blood Observational Study Investigators of ANZICS-Clinical Trials Group, Westbrook A, Pettilä V, Nichol A, Bailey MJ, Syres G, et al. Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med 2010;36:1138–1146. [DOI] [PubMed] [Google Scholar]
- 10.Rice TW, Morris S, Tortella BJ, et al. Deviations from evidence-based clinical management guidelines increase mortality in critically injured trauma patients. Crit Care Med 2012;40:778–786. [DOI] [PubMed] [Google Scholar]
- 11.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010;50:1227–123. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix J, Demaret P, Tucci M. Red blood cell transfusion: decision making in pediatric intensive care units. Semin Perinat 2012;36:225–231. [DOI] [PubMed] [Google Scholar]
- 13.Nellis ME, Karam O, Valentine SL, et al. Executive Summary of Recommendations and Expert Consensus for plasma and platelet transfusion practice in critically ill children from the Transfusion and Anemia EXpertise Initiative – Control/Avoidance of Bleeding (TAXI-CAB). Pediatr Crit Care Med to complete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nellis ME, Tucci M, Lacroix J, et al. Bleeding assessment scale in critically ill children (BASIC): Physician-driven diagnostic criteria for bleeding severity. Crit Care Med 2019;47:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murshid WR, Gader AG. The coagulopathy in acute head injury: Comparison of cerebral versus peripheral measurements of haemostatic activation markers. Br J Neurosurg 2002;16:362–369. [DOI] [PubMed] [Google Scholar]
- 17.Miner ME, Kaufman HH, Graham SH, et al. Disseminated intravascular coagulation fibrinolytic syndrome following head injury in children: Frequency and prognostic implications. J Pediatr 1982;100:687–691. [DOI] [PubMed] [Google Scholar]
- 18.Frith D, Brohi K. The pathophysiology of trauma-induced coagulopathy. Curr Opin Crit Care 2012;18:631–636. [DOI] [PubMed] [Google Scholar]
- 19.Kinslow K, McKenney M, Boneva D, et al. Massive transfusion protocols in paediatric trauma population: A systematic review. Transfus Med 2020;30:333–342. [DOI] [PubMed] [Google Scholar]
- 20.Neff LP, Cannon JW, Morrison JJ, et al. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg 2015;78:22–28. [DOI] [PubMed] [Google Scholar]
- 21.Butler EK, Mills BM, Arbabi S, et al. Association of blood component ratios with 24-hour mortality in injured children receiving massive transfusion. Crit Care Med 2019;47:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham ME, Rosenfeld EH, Zhu H, et al. A high ratio of plasma: RBC improves survival in massively transfused injured children. J Surg Res 2019; 233:213–220. [DOI] [PubMed] [Google Scholar]
- 23.Noland DK, Apelt N, Greenwell C, et al. Massive transfusion in pediatric trauma: An ATOMAC perspective. J Pediatr Surg 2019;54:345–349. [DOI] [PubMed] [Google Scholar]
- 24.Nosanov L, Inaba K, Okoye O, et al. The impact of blood product ratios in massively transfused pediatric trauma patients. Am J Surg 2013;206:655–660. [DOI] [PubMed] [Google Scholar]
- 25.Edwards MJ, Lustik MB, Clark ME, et al. The effects of balanced blood component resuscitation and crystalloid administration in pediatric trauma patients requiring transfusion in Afghanistan and Iraq 2002 to 2012. J Trauma Acute Care Surg 2015;78:330–335. [DOI] [PubMed] [Google Scholar]
- 26.Snyder CW, Weinberg JA, McGwin G, et al. The relationship of blood product ratio to mortality: Survival benefit or survival bias? J Trauma 2009;66:358–362. [DOI] [PubMed] [Google Scholar]
- 27.Cannon JW, Johnson MA, Caskey RC, et al. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg 2017;83:211–217. [DOI] [PubMed] [Google Scholar]
- 28.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg 2010;210:957–965. [DOI] [PubMed] [Google Scholar]
- 29.Valentine SL, Bateman ST, Bembea MM, et al. Consensus recommendations for red blood cell transfusion practice in critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI). Pediatr Crit Care Med 2018;19:884–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karam O, Russell RT, Stricker P, et al. , on behalf of the Pediatric Critical Care Transfusion and Anemia Expertise Initiative: Recommendations on red blood cell transfusions in critically ill children with non-life threatening or life-threatening bleeding from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI). Pediatr Crit Care Med 2018;19(Suppl 9):S127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinella PC, Perkins JG, Grathwohl KW, et al. Fresh whole blood transfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a U.S. combat support hospital. World J Surg 2008;32:2–6. [DOI] [PubMed] [Google Scholar]
- 32.Vanderspurt CK, Spinella PC, Cap AP, et al. The use of whole blood in US military operations in Iraq, Syria, and Afghanistan since the introduction of low-titer Type O whole blood: feasibility, acceptability, challenges. Transfusion 2019;59:965–970. [DOI] [PubMed] [Google Scholar]
- 33.Spinella PC, editor. Damage control resuscitation: identification and treatment of life-threatining hemorrhage. Berlin: Springer; 2020. [Google Scholar]
- 34.Liras IN, Caplan HW, Stensballe J, et al. Prevalence and impact of admission acute traumatic coagulopathy on treatment intensity, resource use, and mortality: An evaluation of 956 severely injured children and adolescents. J Am Coll Surg 2017;224:625–632. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham AJ, Condron M, Schreiber MA, et al. Rotational thromboelastometry (ROTEM) predicts transfusion and disability in pediatric trauma. J Trauma Acute Care Surg 2020;88:134–140. [DOI] [PubMed] [Google Scholar]
- 36.Vogel AM, Radwan ZA, Cox CS, et al. Admission rapid thrombelastography delivers real-time “actionable” data in pediatric trauma. J Pediatr Surg 2013;48:1371–1376. [DOI] [PubMed] [Google Scholar]
- 37.Hendrickson JE, Shaz BH, Pereira G, et al. Coagulopathy is prevalent and associated with adverse outcomes in transfused pediatric trauma patients. J Pediatr 2012;160:204–209.e3. [DOI] [PubMed] [Google Scholar]
- 38.Patregnani JT, Borgman MA, Maegele M, et al. Coagulopathy and shock on admission is associated with mortality for children with traumatic injuries at combat support hospitals. Pediatr Crit Care Med 2012;13:273–277. [DOI] [PubMed] [Google Scholar]
- 39.Leeper CM, Kutcher M, Nasr I, et al. Acute traumatic coagulopathy in a critically injured pediatric population: Definition, trend over time, and outcomes. J Trauma Acute Care Surg 2016;81:34–41. [DOI] [PubMed] [Google Scholar]
- 40.Deng Q, Hao F, Wang Y, Guo C. Rotation thromboelastometry (ROTEM) enables improved outcomes in the pediatric trauma population. J Int Med Res 2018;46:5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afshari A, Wikkelsø A, Brok J, et al. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev 2011;16(3):CD007871. [DOI] [PubMed] [Google Scholar]
- 42.Wikkelsø A, Wetterslev J, Møller AM, et al. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev 2016;22(8):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Y, Hei F, Long C, et al. Perioperative monitoring of thromboelastograph on blood protection and recovery for severely cyanotic patients undergoing complex cardiac surgery. Artificial Organs 2010;34;34:955–960. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama Y, Nakajima Y, Tanaka KA, et al. Thromboelastometry‐guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth 2015;114:91–102. [DOI] [PubMed] [Google Scholar]
- 45.Baksaas-Aasen K, Gall LS, Stensballe J, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med 2021;47:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leeper CM, Neal MD, Billiar TR, et al. Overresuscitation with plasma is associated with sustained fibrinolysis shutdown and death in pediatric traumatic brain injury. J Trauma Acute Care Surg 2018;85:12–17. [DOI] [PubMed] [Google Scholar]
- 47.Rowell SE, Barbosa RR, Lennox TC, et al. Moderate elevations in international normalized ratio should not lead to delays in neurosurgical intervention in patients with traumatic brain injury. J Trauma Acute Care Surg 2014;77:846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer DF, McGwin G Jr, Melton SM, et al. The relationship between INR and development of hemorrhage with placement of ventriculostomy. J Trauma 2011;70:1112–1117. [DOI] [PubMed] [Google Scholar]
- 49.Karam O, Demaret P, Duhamel A, et al. Factors influencing plasma transfusion practices in paediatric intensive care units around the world. Vox Sang 2017;112:140–149. [DOI] [PubMed] [Google Scholar]
- 50.American Society of Anesthesiologists Task Force on Perioperative Blood Management: Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015;122:241–275. [DOI] [PubMed] [Google Scholar]
- 51.Barnard D, Portwine C, and Members of the C17. Standards and Guidelines Group: Guideline for platelet transfusion thresholds for pediatric hematology/oncology patients. In: C17 Council, Children’s Cancer & Blood Disorders, editors.; Edmonton. Edmonton: 2010. [Google Scholar]
- 52.Davis JW, Davis IC, Bennink LD, et al. Placement of intracranial pressure monitors: are “normal” coagulation parameters necessary? J Trauma 2004;57:1173–1177. [DOI] [PubMed] [Google Scholar]
- 53.Karam O, Demaret P, Shefler A, et al. Indications and effect of plasma transfusions in critically ill children. Am J Respir Crit Care Med 2015;191:1395–1402. [DOI] [PubMed] [Google Scholar]
- 54.Karam O, Spinella P, Wong E, et al. Blood products and transfusion therapy. In: Shaffner DH, Nichols DG, editor. Rogers Textbook of Pediatric Intensive Care. 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 55.Josephson CD, Granger S, Assmann SF, et al. Bleeding risks are higher in children versus adults given prophylactic platelet transfusions for treatment-induced hypoproliferative thrombocytopenia. Blood 2012;120:748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leeper CM, Yazer MH, Cladis FP, et al. Cold-stored whole blood platelet function is preserved in injured children with hemorrhagic shock. J Trauma Acute Care Surg 2019;87:49–53. [DOI] [PubMed] [Google Scholar]
- 57.Polites SF, Nygaard RM, Reddy PN, et al. Multicenter study of crystalloid boluses and transfusion in pediatric trauma-When to go to blood? J Trauma Acute Care Surg 2018;85:108–112. [DOI] [PubMed] [Google Scholar]
- 58.Fahy AS, Thiels CA, Polites SF, et al. Prehospital blood transfusions in pediatric trauma and nontrauma patients: a single-center review of safety and outcomes. Pediatr Surg Int 2017;33:787–792. [DOI] [PubMed] [Google Scholar]
- 59.Hwu RS, Keller MS, Spinella PC, et al. Potential effects of high plasma to red blood cell ratio transfusion in pediatric trauma. Trauma 2017;19:21–27. [Google Scholar]
- 60.Du Pont-Thibodeau G, Lacroix J, et al. Platelet transfusions in pediatric intensive care. Pediatr Crit Care Med 2016;17:e420–429. [DOI] [PubMed] [Google Scholar]
- 61.Hwu RS, Spinella PC, Keller MS, et al. The effect of massive transfusion protocol implementation on pediatric trauma care. Transfusion 2016;56:2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noorman F, van Dongen TT, Plat MJ, et al. Transfusion: −80 degrees C frozen blood products are safe and reffective in military casualty care. PLoS One 2016;11:e0168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson KL, Schenarts PJ, Bacchetta MD, et al. Pediatric trauma experience in a combat support hospital in eastern Afghanistan over 10 months, 2010 to 2011. Am Surg 2013;79:257–260. [PubMed] [Google Scholar]
- 64.Hendrickson JE, Shaz BH, Pereira G, et al. Implementation of a pediatric trauma massive transfusion protocol: one institution’s experience. Transfusion 2012;52:1228–1236. [DOI] [PubMed] [Google Scholar]
- 65.Inaba K, Branco BC, Rhee P, et al. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma 2011;71:1766–1773. [DOI] [PubMed] [Google Scholar]
- 66.Inaba K, Branco BC, Rhee P, et al. Impact of ABO-identical vs ABO-compatible nonidentical plasma transfusion in trauma patients. Arch Surg 2010;145:899–906. [DOI] [PubMed] [Google Scholar]
- 67.Alsheikh B, Chegondi M, Totapally B. Platelet Transfusion Thresholds Among Children Admitted to a Pediatric Intensive Care Unit. Cureus 2017; 9:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nadler R, Mozer-Glassberg Y, Gaines B, Glassberg E, Chen J. Freeze dried plasma for the resuscitation of traumatized pediatric patients: Response. J Trauma Acute Care Surg 2020; 88:e152–e153. [DOI] [PubMed] [Google Scholar]
- 69.Livingston MH, Singh S, Merritt NH. Massive transfusion in paediatric and adolescent trauma patients: incidence, patient profile, and outcomes prior to a massive transfusion protocol. Injury 2014; 45:1301–6. [DOI] [PubMed] [Google Scholar]
- 70.Murphy CH, Spain DA, Shan H. Coagulopathy and transfusion ratios in pediatric trauma. J Trauma Acute Care Surg 2020; 88:648–653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.