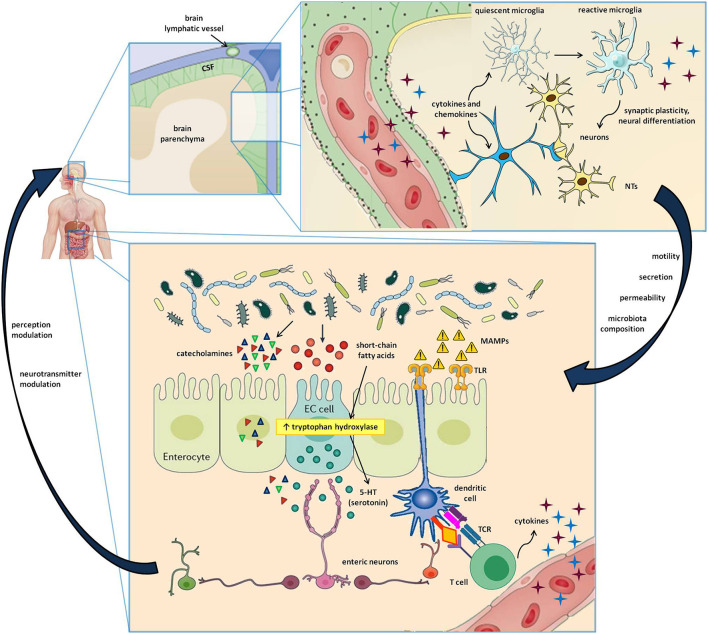
Figure 1.
Cells and molecules involved in the gut-brain-immune axis. The lower box represents the intestinal microenvironment, where microbial niches interact with the gut mucosa by directly secreting active metabolites or by indirectly stimulating the release of neurotransmitters by enteroendocrine cells (EC cells). Furthermore, the microbe-associated molecular patters (MAMPs) stimulate Toll-like receptors (TLR) on enterocytes and dendritic cells, resulting in innate and adaptive immune triggers. The upper box shows a magnification of the cerebral parenchima—blood-brain barrier interface, where neurons, astrocytes, microglial cells, and immune cells carried by brain lymphatics all take part in a well-coordinated interplay. In addition to the signals provided by circulating molecules acting in a paracrine and endocrine fashion, the bidirectional gut-brain interaction includes afferent and efferent neural pathways also involving the autonomous and the enteric nervous systems, resulting in modulation of perception, gut motility, secretion, mucosal permeability, and even changes in the microbial ecosystem.