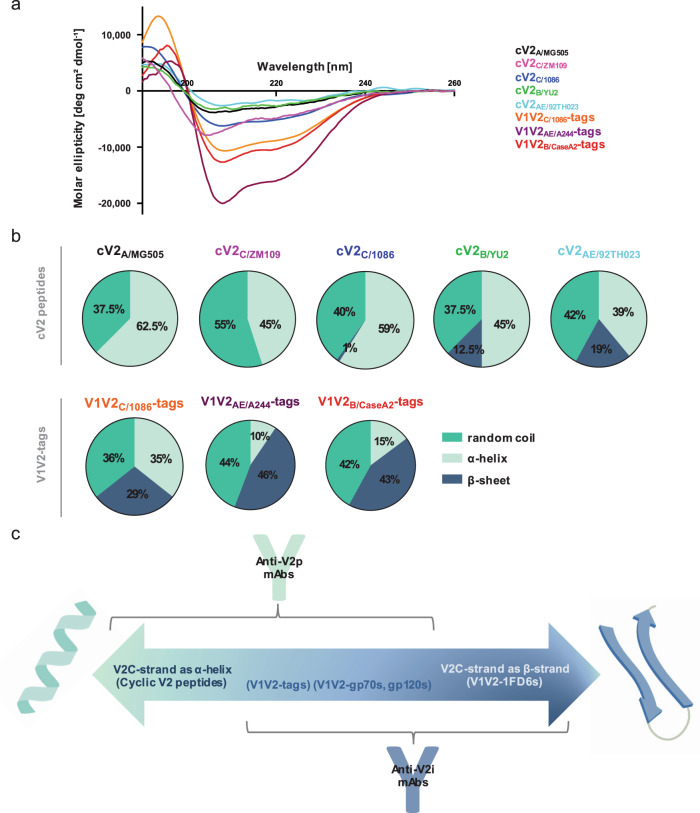
Fig. 2. Structural conformations of the V1V2 domain.
a Circular dichroism spectra of five cyclic V2 peptides and three V1V2-tags proteins. b Pie charts depicting the percentage of secondary structure (random coil [turquoise], α-helix [light green], or β-sheet [dark blue]) determined by the circular dichroism spectra and calculated by CD-FIT software (https://www.ruppweb.org/Xray/comp/cdfit.htm). c Schematic diagram created with BioRender.com showing the alterative structures of the V2 C-strand (V2C) between an α-helix (left) and a β-strand (right). According to the proportion of each molecule present in the two conformations, the antigens will react preferentially with V2p Abs (left), V2i Abs (right), or both (middle). Abbreviations as in Fig. 1.