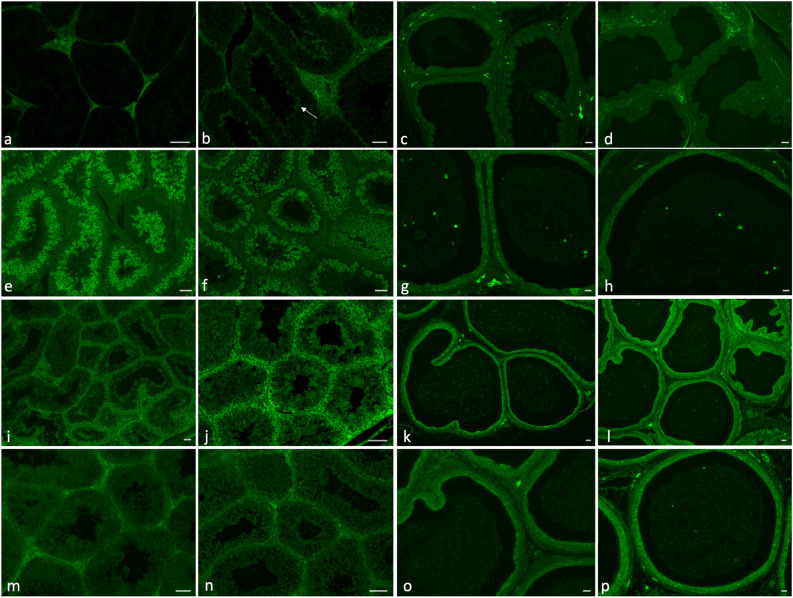
Figure 2.
Immunolocalization of Δ6-desaturase (FADS2) in testis (a,b) and epididymis (c,d); very long-chain fatty acid 5 elongase (ELOVL5) in testis (e,f) and epididymis (g,h); Δ5-desaturase (FADS1) in testis (i,j) and epididymis (k,l); and very long-chain fatty acid 2 elongase (ELOVL2) in testis (m,n) and epididymis (o,p). A high labelling intensity was evident in the interstitial tissue in testis from rabbits fed control (a) and FLAX diets (b); in the latter, the FADS2 signal was present also in elongated spermatids (arrow). In the cauda epididymis (control c, FLAX d), FADS2 was absent from the principal cells; FLAX diet increased the presence of the enzyme in interstitial connective tissue. A high labelling intensity of ELOVL5 was shown in spermatocytes and round spermatids in testis from rabbits fed control (e) and FLAX diets (f). In cauda epididymis control and FLAX (g, h respectively), ELOVL5 appeared in principal epidydimal cells, in interstitial connective tissue and in numerous vesicles in the lumen where the spermatozoa were located. A clear signal of FADS1 was detected in Sertoli cells in testis from rabbits fed control (i) and FLAX diets (j); in the latter, the fluorescence intensity was increased and present in elongated spermatids. In the cauda epididymis, both control and FLAX (k, l), FADS1 was localized in interstitial connective tissue, basal and principal epididymal cells, and in a very large number of epididymal vesicles. A high labelling intensity of ELOVL2 was evident in the interstitial tissue in testis from rabbits fed control (m) and FLAX diets (n). In the cauda epididymis (o, p), ELOVL2 appeared localized in basal and principal epididymal cells as well as in interstitial connective tissue; a small number of vesicles appeared fluorescent in the lumen where the spermatozoa were located (more evident in FLAX diet). Bar: 50 µm.