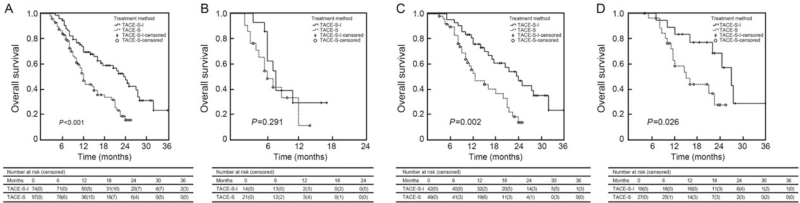
Figure 1.
Kaplan-Meier analyses of survival according to treatment group. (A) For the entire study population, the median OS was 23.5 (95% CI 19.1–27.9) and 12.0 (95% CI 10.7–13.3) months in the TACE-S-I group and TACE-S group, respectively. (B) For patients with type A PVTT, the median OS was 8.0 (95% CI 6.3–9.7) and 6.5 (95% CI 4.1–8.9) months in the TACE-S-I group and TACE-S group, respectively. (C) For patients with type B PVTT, the median OS was 24.0 (95% CI 18.7–29.3) and 12.5 (95% CI 7.8–17.2) months in the TACE-S-I group and TACE-S group, respectively. (D) For patients with type C PVTT, the median OS was 27.0 (95% CI 21.3–32.7) and 15.0 (95% CI 9.3–20.7) months in the TACE-S-I group and TACE-S group, respectively. CI: Confidence interval; OS: Overall survival; PVTT: Portal vein tumor thrombus; TACE-S: Transarterial chemoembolization combined with sorafenib; TACE-S-I: Transarterial chemoembolization combined with sorafenib and 125I seed brachytherapy.