Abstract
Background:
Female breast cancer (FBC) has become the most prevalent malignancy worldwide. We aimed to evaluate the global and regional burden in epidemiological trends and factors associated with the incidence and mortality of FBC.
Methods:
FBC incidence and mortality in 60 selected countries by cancer registry data integrity in 2020 were estimated from the GLOBOCAN database, and their association with the human development index (HDI) was further evaluated. Trends of age-standardized rates of incidence and mortality in 60 countries from 2000 through 2019 were evaluated by joinpoint regression analysis using data of Global Burden of Disease 2019. The association between potential behavioral, metabolic, and socioeconomic risk factor exposure at the nation level retrieved from the World Bank and Global Health Observatory and the incidence and mortality of FBC were evaluated by multivariate linear regression.
Results:
FBC incidence and mortality varied greatly in the 60 included countries. Higher incidence and mortality rates were typically observed in countries with higher HDIs and vice versa. During 2000 to 2019, significantly increasing trends in incidence and mortality were observed in 26 (average annual percent changes [AAPCs], 0.35–2.96) and nine countries (AAPC, 0.30–1.65), respectively, while significantly decreasing trends in both incidence and mortality were observed in 22 countries, most of which were high-HDI countries. Among the population aged ≥40 years, there were 26 and 11 countries showing significantly increased trends in incidence and mortality, respectively. Ecological analysis showed that countries with higher prevalence rates of high cholesterol and higher health expenditures were more likely to have higher FBC incidence, and countries with higher rates of obesity and poorer universal health coverage were more likely to have higher FBC mortality.
Conclusions:
Despite decreased or stabilized FBC incidence and mortality rates were observed in some countries with high HDI over the past decades, disease burden became even severer in developing countries, especially for the population aged ≥40 years. Effective targeted preventive programs are strongly encouraged to reduce the FBC disease burden worldwide.
Keywords: Breast cancer, Trend, Incidence, Mortality, Risk factor
Introduction
Breast cancer is one of the leading malignancies among women, which has caused significant morbidity, and leads to tremendous burden on the healthcare system worldwide. According to a recently released report on GLOBOCAN 2020,[1] there are 2.3 million newly diagnosed cases in 2020, accounting for 11.7% of all newly diagnosed cancer cases. Breast cancer has also been reported to cause 684,996 cancer-related deaths. In 2020, the age-standardized incidence rate (ASIR) and the age-standardized mortality rate (ASMR) of breast cancer were ranked first and second among all cancer types, respectively. The incidence of breast cancer is typically higher in high-income countries.[1] However, in the past decade, it has increased in previous low-incidence countries,[2] such as China, which may be attributed to aging, economic transition, and increased exposure to breast cancer-related risk factors.[3] Therefore, to better develop and initiate strategies for breast cancer prevention and control, understanding the global trends in incidence and mortality and risk factors for breast cancer is crucial.
Primary prevention targeting risk factors associated with breast cancer has great potential to reduce its incidence. The current epidemiological research has identified several modifiable risk factors for breast cancer, including obesity, physical inactivity, consumption of high-protein diet, and alcohol drinking, which can be targeted by primary prevention initiatives.[4] In addition, reproductive factors including early menarche and a short period of breastfeeding were demonstrated to be positively associated with the elevated risk of breast cancer.[5] Apart from these established individual-level risk factors, nation-level socioeconomic factors such as gross domestic product (GDP) and health expenditure may also have an impact on the incidence and mortality of cancer. For instance, a study conducted in European Union countries showed that higher GDP and health expenditure were associated with better cancer prognosis.[6] However, relevant studies on breast cancer are relatively scarce.
Although previous studies have also analyzed trends in breast cancer incidence and mortality, these studies have certain limitations, such as outdated data (up to 2016),[7] coverage of a limited number of countries,[8] and lack of detailed stratification by age.[9] In addition, there is a paucity of research on the impact of national-level factors on breast cancer incidence and mortality. Since the current Cancer Incidence in Five Continents series I-X (CI5) database was last updated in 2012, we used the Global Burden of Disease (GBD) database as a source of data on breast cancer incidence and mortality in different countries, which has been confirmed as a valid source to assess the disease burden data in previous studies.[10]
Therefore, this study aimed to explore global trends in the incidence and mortality of breast cancer using up-to-date data from the GBD 2019.[11] We also aimed to examine the potential correlation between population-based exposure to socioeconomic factors at the national level and the incidence or mortality of breast cancer.
Methods
Data on female breast cancer incidence and mortality
The incidence and mortality of female breast cancer (FBC) in 184 countries in 2020 were obtained from the GLOBOCAN database.[12] We used publicly available data from the GBD Data Resources[11] to assess dynamic trends in FBC incidence and mortality during the past two decades. The GBD database is one of the most important databases for understanding the global burden of disease, and it provides the most comprehensive coverage of the health burden of disease, risk, death, and disease-related disability. Detailed information regarding the GBD database has been provided in previous studies.[13,14] To ensure data quality, we selected representative countries based on the following eligibility criteria: (1) the country vital registration data that were assessed as 3-star or a higher level[13] and (2) the country which had complete incidence and mortality data from 2010 to 2019. In total, 204 countries had GBD data resources, and based on these criteria, 60 countries were ultimately included in this study.
Data on human development index and nation-level risk factor exposure
The human development index (HDI) in 2019 for each included country, which was determined by income, period of education, and life expectancy, was extracted from the United Nations Development Programme.[15] HDI data were divided into four groups according to the quartiles of the distribution of component indicators from 2004 to 2013: low (<0.554), medium (0.554–0.703), high (0.703–0.796), and very high (>0.796).
Several behavioral (alcohol, smoking, physical inactivity), metabolic (obesity, high cholesterol), and socioeconomic (education, unemployment, out-of-pocket GDP, health expenditure, health system, universal health coverage, breast cancer screening) risk factors exposed at the nation level were obtained from the database released by the Global Health Observatory[16] and the World Bank.[17] Detailed definitions of the factors and data sources are listed in the supplementary file [Supplementary Table 1].
Statistical analysis
Females of all ages were included in our study, and they were categorized by 5-year intervals from 0 to 84 years, and those who aged 85 years or older were defined as a separate group. We used Segi world standard population to age standardize the incidence and mortality data, which would reduce the influence of diversity in age distribution. ASIR and ASMR were plotted for each country grouped by the HDI level. The generalized additive model was used to explore the associations.
Trends in incidence/mortality in the included countries were analyzed using the joinpoint regression program (version 4.8.0.1, National Cancer Institute, Bethesda, America) to obtain the annual percentage change (APC) and the average annual percentage change (AAPC), stratified by age (all ages, <40 years and ≥40 years). The APC represents a fixed percentage change in cancer incidence from the previous year and is calculated by fitting a natural log-weighted least squares regression line.[18] The AAPC is a geometric weighted average of APCs within the prespecified time period from the joinpoint analysis, allowing a more concise and visual description of the multi-year changes compared with APCs.[19] Regarding trends, the terms “increasing” or “decreasing” were used when the AAPC was statistically significant (P < 0.05); otherwise, the term “stable” was used. We set a maximum of three joinpoints as the analysis option.
The association between different risk factors and FBC incidence/mortality was analyzed using a multifactorial linear regression model. In addition, the time-lagged effects of risk factors for more than 1 to 4 years were tested. P < 0.05 was considered statistically significant.
Results
Breast cancer incidence and mortality rates in 2020
It was estimated that 226,419 new cases of FBC were diagnosed in 2020, with a global crude incidence rate of 58.5/100,000 and an ASIR of 47.8/100,000 of the population. The top five countries with the highest incidence were from Europe, with Belgium ranking first with an ASIR of 113.2/100,000, followed by the Netherlands with an ASIR of 100.9/100,000 of the population. Countries with low incidence rates were mostly in Africa, among which the ASIR of the Republic of Gambia was only 11/100,000 of the population. The incidence varied considerably among countries in Oceania, the Americas, and Asia. For example, among Asian countries, Israel had the highest ASIR (78.3/100,000), followed by Singapore (77.9/100,000), and Bhutan had the lowest ASIR of 5/100,000 of the population worldwide. China had an ASIR of 39.1/100,000 of the population, and the number of new FBC cases was 416,371, ranking first among all countries.
There were 515,637 deaths from breast cancer in 2020, with an overall ASMR of 11/100,000 of the population. Slovakia had the highest ASMR (29.6/100,000), followed by Hungary (29/100,000). Mortality rates were relatively low in most African countries, such as Comoros (2.3/100,000) and Botswana (2.5/100,000), and varied widely in Oceania, the Americas, and Asia. For instance, the ASMR was 19.8/100,000 of the population in Singapore and only 2.6/100,000 of the population in Bangladesh. The number of deaths in China was 164,959, ranking first among all countries, accounting for 32% of the total number of deaths due to FBC.
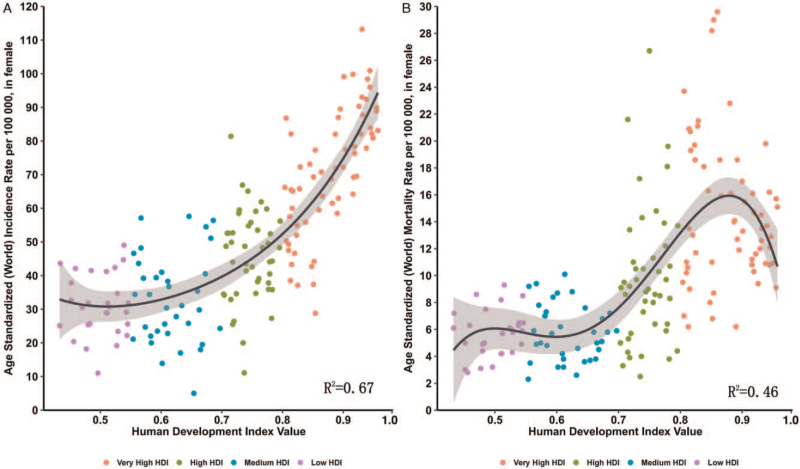
We evaluated the association between HDI levels and the ASIR and ASMR of breast cancer in different countries. Overall, the highest incidence rates were observed in the very high HDI group, and the lowest occurred in the very low HDI group [Figure 1A]. The mortality rate initially increased with the increase in HDI but reached a peak and started to decrease in countries with a very high HDI (>0.9) [Figure 1B].
Figure 1.
Correlation between age-standardized breast cancer incidence (A) and mortality rates (B) and HDI (Data source: GLOBOCAN 2020). ASIR: Age-standardized incidence rate; ASMR: Age-standardized mortality rate; HDI: Human development index.
Trends of FBC incidence and mortality in 2000 to 2019
According to the observed trends of incidence and mortality from 2000 to 2019, we divided the 60 countries into six groups [Table 1]. More detailed data are shown in [Figure 2 and Supplementary Table 2 and 3].
Table 1.
Group categories for trends in breast cancer incidence and mortality in 2010 to 2019 for females, all ages.
| Group | Incidence | Mortality | Countries |
| A | Increase | Increase | Antigua and Barbuda, Barbados, Ecuador, Fiji, Grenada, Guatemala, Mauritius, Mexico, Panama |
| B | Increase | Stable | China, Costa Rica, Cuba, Japan, Philippines, the Republic of Korea, Serbia |
| C | Increase | Decrease | Chile, Colombia, Estonia, Latvia, Poland, Puerto Rico, Romania, Singapore, Slovakia, Trinidad and Tobago |
| D | Stable | Stable | The Republic of Moldova, Saint Lucia |
| E | Stable | Decrease | Argentina, Brazil, Croatia, Finland, Greece, Guyana, Kuwait, Lithuania, the Netherlands, New Zealand |
| F | Decrease | Decrease | Australia, Austria, Belgium, Canada, Denmark, France, Germany, Hungary, Iceland, Israel, Italy, Kyrgyzstan, Luxembourg, Malta, Norway, Slovenia, Spain, Sweden, Switzerland, United Kingdom, United States of America, Uruguay |
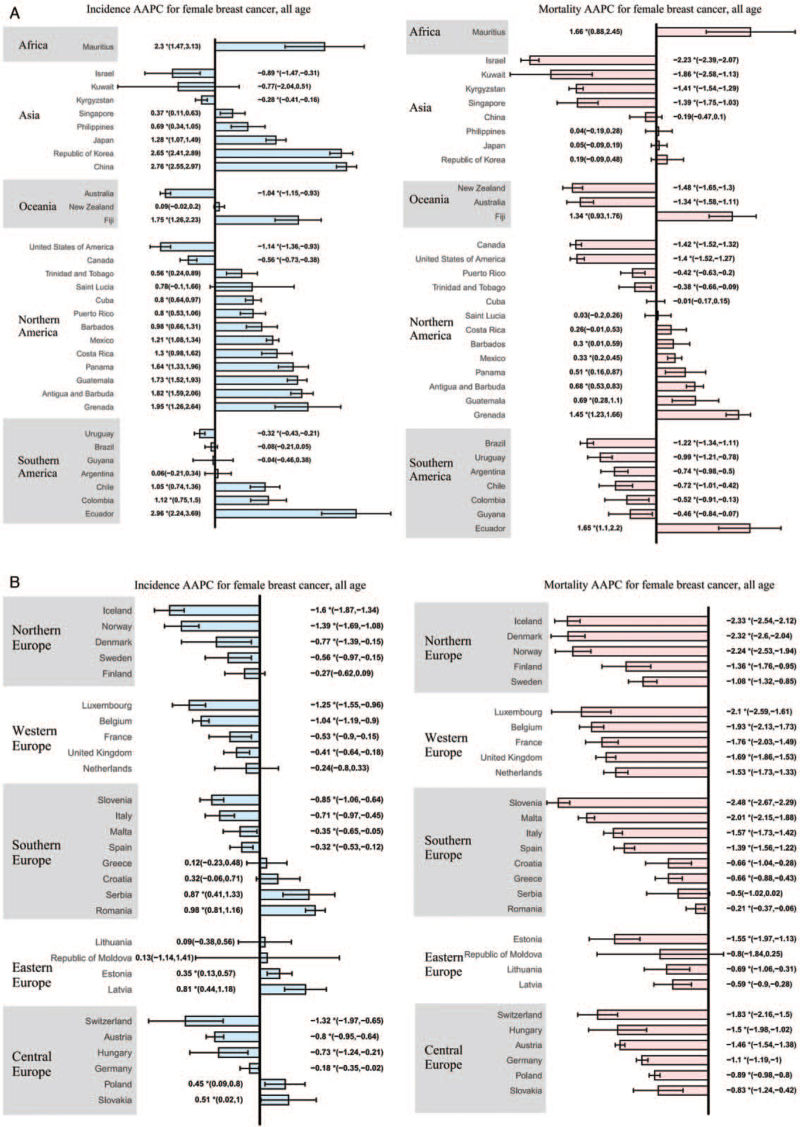
Figure 2.
The AAPC of the incidence and mortality of breast cancer in female, all ages. (A) AAPC for countries in Africa, Asia, Oceania, North America, South America; (B) AAPC for countries in Northern, Southern, Western, Eastern, and Central Europe. ∗P < 0.05; AAPC: Annual percentage change.
In total, 26 countries showed an increasing trend in incidence, with the AAPCs ranging from 0.35 (Estonia) to 2.96 (Ecuador), ten of which were in North America and five in Asia. Ecuador had the greatest increase in incidence (AAPC, 2.96, 95% confidence interval [CI]: 2.24–3.69), followed by China (AAPC, 2.76, 95% CI: 2.55–2.97). Nine of these 26 countries also showed increasing trends in mortality, ranging from 0.30 (Barbados) to 1.66 (Mauritius). The largest increase in mortality was observed in Mauritius (AAPC, 1.66, 95% CI: 0.88–2.45; Table 1, Group A). Ten of the 26 countries presented decreasing mortality trends, and the most significant reduction in mortality was observed in Estonia (AAPC, −1.55, 95% CI: −1.97 to −1.13; Table 1, Group C). Stable mortality rates were observed in four Asian countries (China, Japan, Republic of Korea, and Philippines), Costa Rica, Cuba, and Serbia [Table 1, Group B].
Two countries had stable incidence and mortality rates, including the Republic of Moldova and Saint Lucia [Table 1, Group D]. In ten countries with stable incidence and decreasing mortality, Kuwait had the most notable decrease in mortality (AAPC, −1.86, 95% CI: −2.58 to −1.13; Table 1, Group E).
Decreasing incidence and mortality were observed in 22 of 60 countries, most of which were high-HDI countries such as the United States, United Kingdom, and most Central, Western, and Northern European countries. Iceland had the most significant reduction in incidence (AAPC, −1.60, 95% CI: −1.87 to −1.34), and Slovenia had the most significant decrease in mortality (AAPC, −2.48, 95% CI: −2.67 to −2.29).
Incidence and mortality trends among females aged younger and older than 40 years
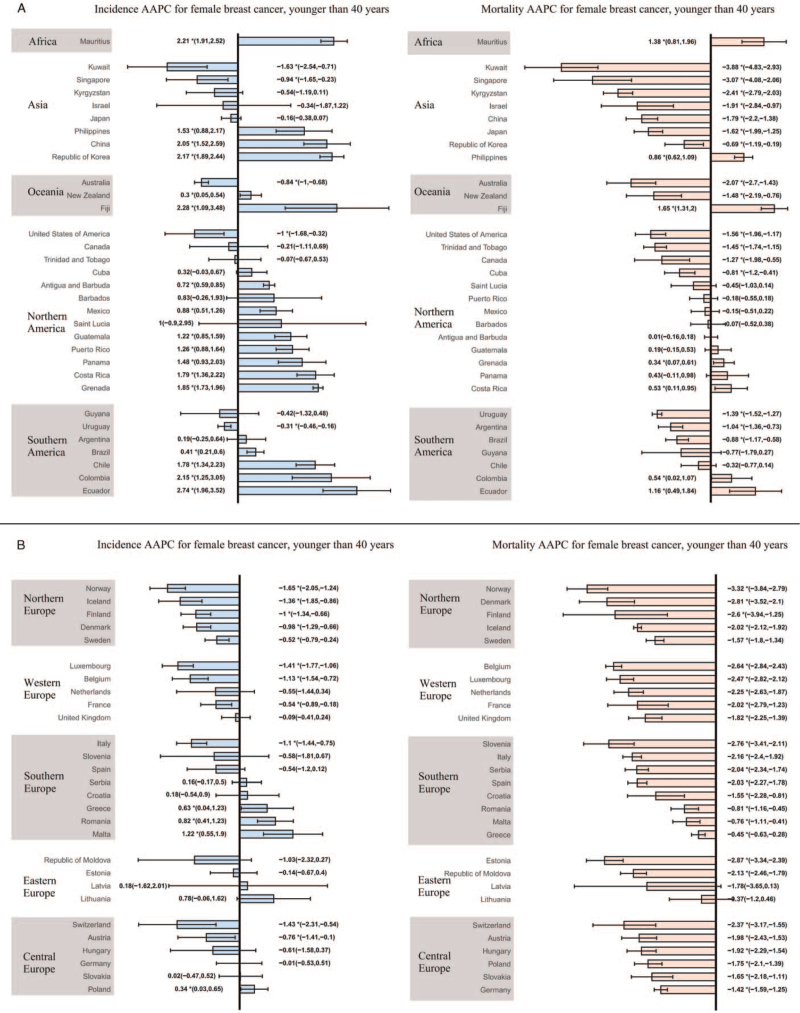
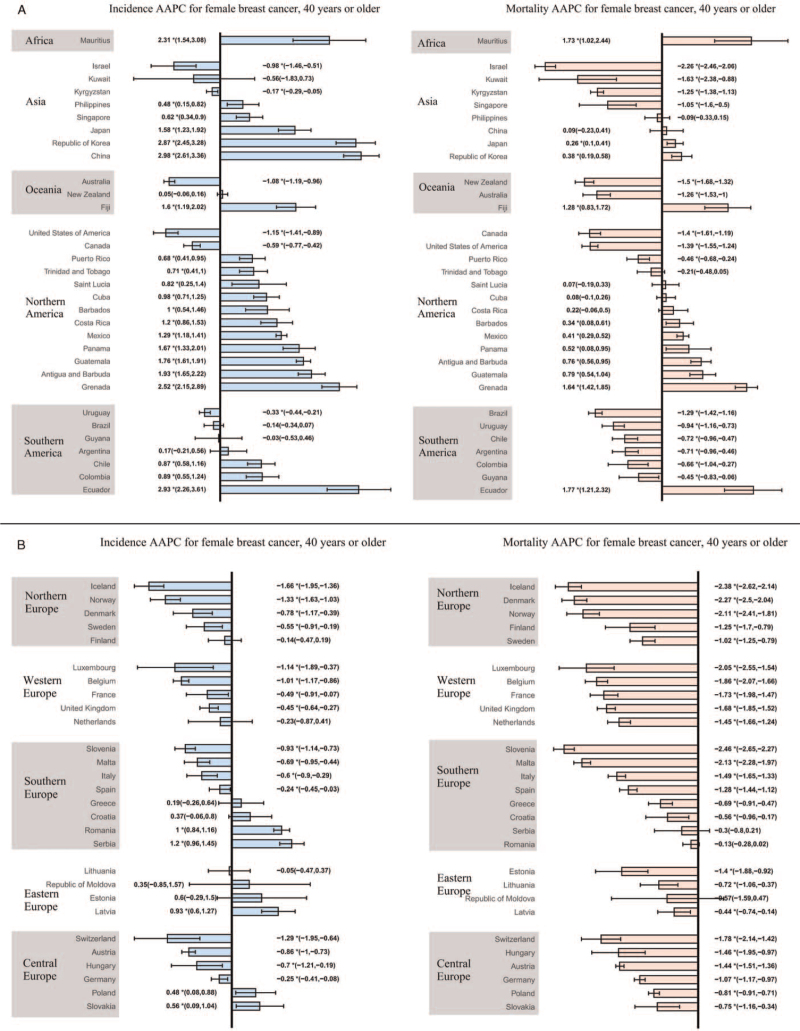
Among females aged younger than 40 years, 21 countries showed an increasing trend in the incidence of FBC. Ecuador (AAPC, 2.74, 95% CI: 1.96–3.52), Fiji (AAPC, 2.28, 95% CI: 1.09–3.48), and Mauritius (AAPC, 2.21, 95% CI: 1.91–2.52) had the most significant increases. Sixteen countries presented a decreasing trend, with Norway (AAPC, −1.65, 95% CI: −2.05 to −1.24) showing the largest decrease, followed by Kuwait (AAPC, −1.63, 95% CI: −2.54 to −0.71). The remaining 23 countries showed a stable trend. As for females aged ≥40 years, the incidence rates of breast cancer were on the rise in 26 countries, with China (AAPC, 2.98, 95% CI: 2.61–3.36), Ecuador (AAPC, 2.93, 95% CI: 2.26–3.61), and the Republic of Korea (AAPC, 2.87, 95% CI: 2.45–3.28) having the highest increase. A decreasing trend in incidence was observed in 22 countries, with Iceland showing the most pronounced reduction (AAPC, −1.66, 95% CI: −1.95 to −1.36), followed by Norway (AAPC, −1.33, 95% CI: −1.63 to −1.03). The remaining 12 countries showed a stable trend.
Regarding FBC mortality, only seven countries showed an increasing trend among women aged younger than 40 years, while the rest showed a stable or decreasing trend. Fiji (AAPC. 1.65, 95% CI: 1.31–2.00) had the most significant increase in incidence, and Kuwait (AAPC, −3.88, 95% CI: −4.83 to −2.93) had the most marked decrease in mortality. Among women aged ≥40 years, we observed an increasing mortality trend in 11 countries, with Ecuador (AAPC, 1.77, 95% CI: 1.21–2.32) showing the greatest increase. The remaining countries showed a stable or decreasing trend, with Slovenia (AAPC, −2.46, 95% CI: −2.65 to −2.27) showing the most significant decrease, followed by Iceland (AAPC, −2.38, 95% CI: −2.62 to −2.14). More detailed data are shown in [Figures 3 and 4 and Supplementary Tables 4–7].
Figure 3.
The AAPC of the incidence and mortality of breast cancer in females aged younger than 40 years. (A) AAPC for countries in Africa, Asia, Oceania, North America, South America; (B) AAPC for countries in Northern, Southern, Western, Eastern, and Central Europe. ∗P < 0.05; AAPC: Annual percentage change.
Figure 4.
The AAPC of the incidence and mortality of breast cancer in females aged 40 years or older. (A) AAPC for countries in Africa, Asia, Oceania, North America, South America; (B) AAPC for countries in Northern, Southern, Western, Eastern, and Central Europe. ∗P < 0.05; AAPC: Annual percentage change.
Association between risk factors and incidence/mortality
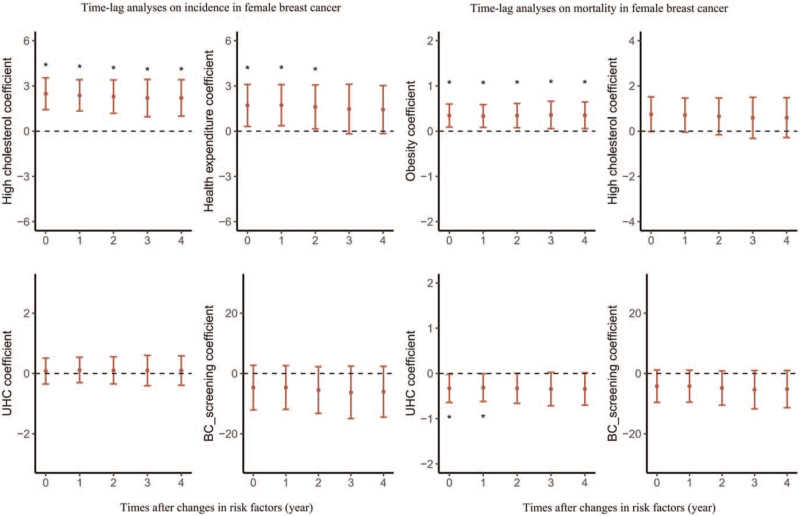
The results of multi-factor linear regression are shown in [Figure 5 and Supplementary Table 8 and 9]. Overall, the higher incidence of FBC was associated with higher prevalence rates of high cholesterol (β = 2.203, P < 0.001) and higher health expenditure (β = 1.608, P = 0.032). Time-lag effect analysis for a 5-year period showed similar results, which are presented in [Figure 5 and Supplementary Table 8].
Figure 5.
Time-lag analyses of changes in risk factors on breast cancer incidence and mortality. BC: breast cancer; UHC: universal health coverage. ∗P < 0.05.
Higher FBC mortality rates were associated with higher obesity rates (β = 0.350, P = 0.020) and poorer universal health coverage (β = −0.314, P = 0.045). Time-lag effect analysis for a 5-year period showed similar results, which are presented in [Figure 5 and Supplementary Table 9].
Discussion
Using up-to-date data from GLOBOCAN 2020 and GBD 2019, this study provided the disease burden and latest trends in the incidence and mortality of breast cancer in 60 countries over the past two decades. Our analysis demonstrated that many high-HDI countries showed a stabilization or even a reduction in incidence and a significant decrease in mortality, while most developing countries presented a tremendous increase in incidence. It was also noteworthy that the incidence of breast cancer in women aged younger than 40 years was significantly increasing in 21 of 60 countries, especially in developing countries. Ecology analysis revealed that exposure to several factors at the national level was associated with higher FBC incidence and mortality, including higher prevalence of a high level of cholesterol, higher obesity rates, and lower rates of universal health coverage. The identified disease pattern and relevant risk factors are important references for implementing appropriate breast cancer prevention initiatives to reduce the overall disease burden.
Our results of trends in FBC incidence showed that high-HDI countries such as the United States of America, Australia, and most countries in Northern, Western, and Central Europe experienced a stabilization or decline in incidence. Several studies have observed similar results of decreasing incidence in high-HDI countries.[9,20,21] There are two possible reasons for this. First, the reduced use of post-menopausal hormone therapy, a combined estrogen-progestin therapy that has been confirmed to increase the risk of FBC, is one possible reason.[22] For instance, in the United States of America, prescriptions for hormone replacement therapy began to fall sharply since 2002, and the incidence of FBC began to decline subsequently, while the prevalence of other risk factors (such as reproductive factors and mammographic screening) did not change significantly.[23–25] Second, FBC screening programs have been widely introduced in these countries. Evidence from observational and randomized controlled trials has demonstrated the effectiveness of screening in reducing the incidence of breast cancer.[26–28]
The rising incidence of FBC in developing countries may be attributed to a series possible reasons, such as the adoption of so-called westernized lifestyle (such as sedentary lifestyle, early menarche, a short period of breastfeeding), increase in alcohol consumption, and exposure to secondhand smoking.[4,29,30] In addition, developing countries are experiencing a switch in the disease spectrum and a rise in life expectancy; therefore, this may lead to increased exposure to breast cancer risk factors.[31] The decline in mortality rates in most middle- and high-income countries is consistent with the widely held belief that economic development will promote advances in medical technology, enhance access to health services, and help prevent and treat diseases.[32,33]
Notably, the incidence of FBC in women aged younger than 40 years was significantly higher in several developing countries in Asia, North America, and South America, which is consistent with the results of several previous studies.[34–37] This phenomenon may be the result of younger women being more susceptible to development-related risk factors, such as delayed childbirth and reduced breastfeeding period. FBC tends to be more pathologically and clinically aggressive and has a poorer prognosis in those who are younger than 40 years than in their older counterparts.[38,39] The treatment of FBC greatly devastates the fertility and menstrual rhythm of younger females.[40] Therefore, breast cancer among females aged younger than 40 years should be taken seriously.
We observed that higher prevalence of a high cholesterol level and higher health expenditure at the national level were associated with higher FBC incidence. Universal health coverage was a protective factor against mortality, while obesity was a risk factor. Previous studies addressing the effect of cholesterol on the risk of FBC incidence have reached inconsistent conclusions, with some suggesting high cholesterol level as a risk factor.[41,42] Countries with higher health expenditures tend to be high-income and developed countries, where the incidence of FBC is also higher; hence, there may be an inverse positive correlation between health expenditures and FBC incidence. The achievement of universal health coverage will help reduce the proportion of out-of-pocket costs in health expenditures and increase women access to many basic health services, including screening, diagnosis, and treatment.[43] This will, to some extent, improve the prognosis of FBC and reduce mortality. Several meta-analyses indicate that obesity is associated with increased risk of FBC recurrence and death,[44] which is consistent with our result.
When interpreting our results, there are several strengths and limitations that need to be considered. The strengths of our analysis include that our study is the latest research on the trends in FBC incidence and mortality during 2000 to 2019. Most importantly, we also explored the association between behavioral, metabolic, and socioeconomic indicators exposed at the national level and the incidence and mortality of FBC. Our study had some limitations. First, the latest incidence and mortality data obtained from GBD were estimated by modeling and not from the cancer registry, which may lead to discrepancies with the actual circumstances. However, many studies have confirmed the validity of GBD data in predicting cancer disease burden.[13,14,45] Second, the ecological fallacy could not be avoided because we only extracted national-level data, and there was no data on the joint distribution of individual exposure and outcomes.
In summary, the present study found that the worldwide burden of breast cancer remains severe. Several high-HDI countries had a stabilization or reduction in incidence and a significant decrease in mortality, while most developing countries have presented a tremendous increase in incidence over the past two decades. Although the disease burden of FBC was more prominent in the population aged ≥40 years, the increased incidence of early-onset breast cancer in younger women aged younger than 40 years in some countries should be drawn attention. Therefore, there is a strong need to implement effective preventive measures, including controlling the associated risk factors and conducting population-based screening programs, to reduce the incidence and mortality of FBC worldwide.
Funding
This work was supported by grants from the Natural Science Foundation of Beijing Municipality (No. 7202169), the Beijing Nova Program of Science and Technology (No. Z191100001119065), and the CAMS Innovation Fund for Medical Sciences (No. 2017-I2M-1-006).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Luo C, Li N, Lu B, Cai J, Lu M, Zhang Y, Chen H, Dai M. Global and regional trends in incidence and mortality of female breast cancer and associated factors at national level in 2000 to 2019. Chin Med J 2022;135:42–51. doi: 10.1097/CM9.0000000000001814
Chenyu Luo and Na Li contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 2004; 6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Wen W, Zheng Y, Gao YT, Wu C, Bao P, et al. Breast cancer incidence and mortality: trends over 40 years among women in Shanghai, China. Ann Oncol 2016; 27:1129–1134. doi: 10.1093/annonc/mdw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter P. “Westernizing” women's risks? Breast cancer in lower-income countries. N Engl J Med 2008; 358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012; 13:1141–1151. doi: 10.1016/s1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ades F, Senterre C, de Azambuja E, Sullivan R, Popescu R, Parent F, et al. Discrepancies in cancer incidence and mortality and its relationship to health expenditure in the 27 European Union member states. Ann Oncol 2013; 24:2897–2902. doi: 10.1093/annonc/mdt352. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990-2016: evidence from Global Burden of Disease Study 2016. Breast Cancer (Tokyo, Japan) 2019; 26:428–445. doi: 10.1007/s12282-018-00941-4. [DOI] [PubMed] [Google Scholar]
- 8.Shin HR, Boniol M, Joubert C, Hery C, Haukka J, Autier P, et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci 2010; 101:1241–1246. doi: 10.1111/j.1349-7006.2010.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990-2017. Breast Cancer Res Treat 2020; 180:481–490. doi: 10.1007/s10549-020-05561-1. [DOI] [PubMed] [Google Scholar]
- 10.Ji P, Gong Y, Jin ML, Hu X, Di GH, Shao ZM. The burden and trends of breast cancer from 1990 to 2017 at the global, regional, and national levels: results from the global burden of disease study 2017. Front Oncol 2020; 10:650.doi: 10.3389/fonc.2020.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Global Burden of Disease Data Resources. Available at http://ghdx.healthdata.org/gbd-results-tool.[Accessed April 15, 2021] [Google Scholar]
- 12. GLOBOCAN database. Available at https://gco.iarc.fr/today/home. [Accessed on April 15, 2021] [Google Scholar]
- 13.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020; 396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattiuzzi C, Lippi G. Cancer statistics: a comparison between World Health Organization (WHO) and Global Burden of Disease (GBD). Eur J Public Health 2020; 30:1026–1027. doi: 10.1093/eurpub/ckz216. [DOI] [PubMed] [Google Scholar]
- 15.Human Development Report 2020. New York: United Nations Development Programme (UNDP). [Google Scholar]
- 16.Global Health Observatory website. Available at https://www.who.int/data/gho. [Accessed April 15, 2021]. [Google Scholar]
- 17.The World Bank website. Available at https://databank.worldbank.org/source. [Accessed April 15, 2021]. [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med 2009; 28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Hu K, Ding P, Wu Y, Tian W, Pan T, Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open 2019; 9:e028461.doi: 10.1136/bmjopen-2018-028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women Health Initiative randomized controlled trial. JAMA 2002; 288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 23.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA 2004; 291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Buist DSM, Newton KM, Miglioretti DL, Beverly K, Connelly MT, Andrade S, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol 2004; 104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 25.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007; 356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 26.Youlden DR, Cramb SM, Dunn NAM, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012; 36:237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 28.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer 2003; 97:1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 29.Shrubsole MJ, Gao YT, Dai Q, Shu XO, Ruan ZX, Jin F, et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer 2004; 110:605–609. doi: 10.1002/ijc.20168. [DOI] [PubMed] [Google Scholar]
- 30.Ronco AL, De Stefani E, Correa P, Deneo-Pellegrini H, Boffetta P, Acosta G, et al. Dietary benzo[a]pyrene, alcohol drinking, and risk of breast cancer: a case-control study in Uruguay. Asian Pac J Cancer Prevent 2011; 12:1463–1467. doi: 10.1097/01.cad.0000390767.85658.83. [PubMed] [Google Scholar]
- 31.Ding Y, Chen X, Zhang Q, Liu Q. Historical trends in breast Cancer among women in China from age-period-cohort modeling of the 1990–2015 breast Cancer mortality data. BMC Public Health 2020; 20:1280.doi: 10.1186/s12889-020-09375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taheri M, Tavakol M, Akbari ME, Almasi-Hashiani A, Abbasi M. Relationship of socio economic status, income, and education with the survival rate of breast cancer: a meta-analysis. Iran J Public Health 2019; 48:1428–1438. doi: 10.18502/ijph.v48i8.2981. [PMC free article] [PubMed] [Google Scholar]
- 33.Masià J, Merchán-Galvis Á, Salas K, Requeijo C, Cánovas E, Quintana MJ, et al. Socio-economic impact on women diagnosed and treated for breast cancer: a cross-sectional study. Clin Transl Oncol 2019; 21:1736–1745. doi: 10.1007/s12094-019-02185-w. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PSY. Spectrum of breast cancer in Asian women. World J Surg 2007; 31:1031–1040. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 35.Park M, Lim J, Lee JA, Park BK, Jung KW, Won YJ, et al. Cancer incidence and survival among adolescents and young adults in Korea: an Update for 2016. Cancer Res Treat 2021; 53:32–44. doi: 10.4143/crt.2020.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev 2005; 14:1986–1990. doi: 10.1158/1055-9965.Epi-04-0932. [DOI] [PubMed] [Google Scholar]
- 37.Carraro DM, Folgueira MAAK, Lisboa BCG, Olivieri EHR, Krepischi ACV, de Carvalho AF, et al. Comprehensive analysis of BRCA1, BRCA2 and TP53 germline mutation and tumor characterization: a portrait of early-onset breast cancer in Brazil. PLoS One 2013; 8:e57581.doi: 10.1371/journal.pone.0057581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008; 26:3324–3330. doi: 10.1200/jco.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 39.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 40.Carneiro MM, Cota AM, Amaral MC, Pedrosa ML, Martins BO, Furtado MH, et al. Motherhood after breast cancer: Can we balance fertility preservation and cancer treatment? A narrative review of the literature. JBRA Assist Reprod 2018; 22:244–252. doi: 10.5935/1518-0557.20180032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray G, Husain SA. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin Biochem 2001; 34:71–76. doi: 10.1016/s0009-9120(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 42.Ferraroni M, Gerber M, Decarli A, Richardson S, Marubini E, de Paulet PC, et al. HDL-cholesterol and breast cancer: a joint study in northern Italy and southern France. Int J Epidemiol 1993; 22:772–780. doi: 10.1093/ije/22.5.772. [DOI] [PubMed] [Google Scholar]
- 43.Kong YC, Wong LP, Ng CW, Taib NA, Bhoo-Pathy NT, Yusof MM, et al. Understanding the financial needs following diagnosis of breast cancer in a setting with universal health coverage. Oncologist 2020; 25:497–504. doi: 10.1634/theoncologist.2019-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol 2016; 34:4203–4216. doi: 10.1200/jco.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Deng Y, Zhou L, Tian T, Yang S, Wu Y, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol 2019; 12:140.doi: 10.1186/s13045-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.