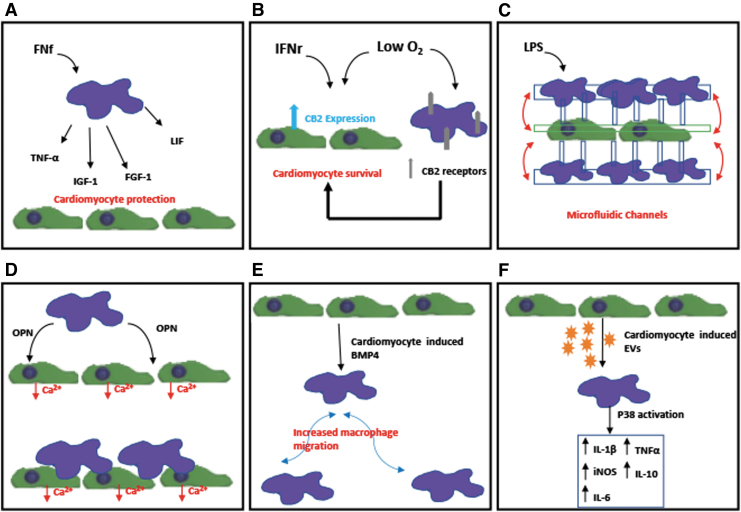
FIG. 2.
Schematics of in vitro studies demonstrating the interaction between cardiomyocytes and macrophages: (A) Demonstration of macrophages activated by FNf-protecting cardiomyocytes from apoptosis through the secretion of TNF-α, FGF-1, IGF-1, and LIF.25 (B) Demonstration of both hypoxia and IFNγ treatment inducing the expression of CB2 receptor in cardiomyocytes and macrophages and that CB2 receptor could play a critical role in the survival of cardiomyocytes post-MI.26 (C) Potential use of microfluidic coculture device for real-time monitoring of interaction of macrophages and cardiac cells.27 (D) Demonstration of both macrophage-derived factors, specifically matricellular protein OPN and direct coupling with macrophages affect calcium-handling function of cardiomyocytes.28 (E) Demonstration of cardiomyocyte-conditioned media causing increased migration of proinflammatory macrophage through BMP4.29 (F) EVs derived from cardiomyocytes promoted a proinflammatory phenotype in macrophages with increased expression of iNOS, IL-1β, IL-6, TNF-α, and IL-10 through activation of the map kinase pathway p38.30 CB2, cannabinoid receptor 2; EVs, extracellular vesicles; FGF-1, fibroblast growth factor-1; FNf, fibronectin fragments; IFNγ, interferon-gamma; IGF-1, insulin-like growth factor 1; iNOS, inducible nitric oxide synthase; LIF, leukemia inhibiting factor; LPS, lipopolysaccharides; OPN, osteopontin; TNF-α, tumor necrosis factor-α.