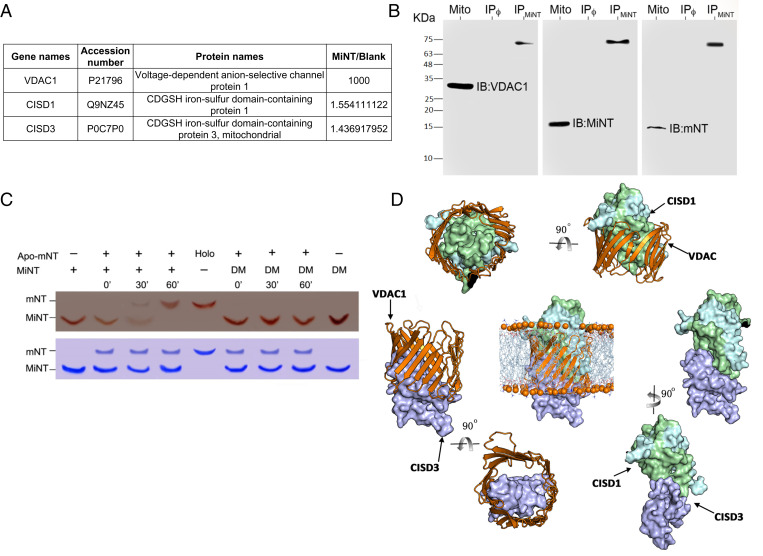
Fig. 3.
MiNT interacts with VDAC and mNT and transfers its [2Fe-2S] cluster to apo-mNT. (A) Identification of VDAC1, mNT, and MiNT proteins following Co-IP with an anti-MiNT antibody applied to an enriched mitochondrial fraction from MDA-MB-231 cells (SI Appendix, Table S1). (B) Western blots of VDAC1/MiNT/mNT after Co-IP of mitochondrial enriched fraction from MDA-MB-231 WT cells, using MiNT, VDAC1, or mNT antibodies. The presence of VDAC1, MiNT, or mNT was detected on the mitochondrial protein extract (Mito), or on protein G beads that were preincubated with MiNT antibody (IPMiNT) or not (IPϕ). (Left) Blot treated with anti-VDAC1 alone, showing the VDAC1 protein band at the molecular weight 30 KDa in the Mito line, (Middle) blot treated with anti-MiNT antibody showing the band in the Mito line at 14 KDa, and (Right) blot treated with anti-mNT antibody showing a specific binding at the Mito line at 12 KDa. All three membranes showed a higher molecular weight band at the same size in the IPMiNT line of about 60 KDa, which indicated the presence of the VDAC1–MiNT–mNT complex. (C) (Upper) Native gel showing the holo-NEET protein as a red band due to the presence of [2Fe-2S] cluster imbedded in the protein. The mNT protein is the upper band, while MiNT protein is the lower band. Holo-MiNT is shown to donate its clusters to apo-mNT after 30 min of incubation with apo-mNT in the presence of a reducing agent. DM is the double-mutated form of MiNT that is very stable and is not able to donate its [2Fe-2S] clusters; it is used as a negative control for the [2Fe-2S] transfer between the two NEET proteins. (Lower) A duplicate gel stained with Coomassie for protein levels. (D) The computationally constructed ternary binding complexes; mNT binds with VDAC1 mainly via the identified site 2 and part of site 1, and uses its large area of site 1 to interact with site1 of MiNT. Besides, site 2 and site 3 of MiNT are the major areas that contribute to the contacts with VADC1.