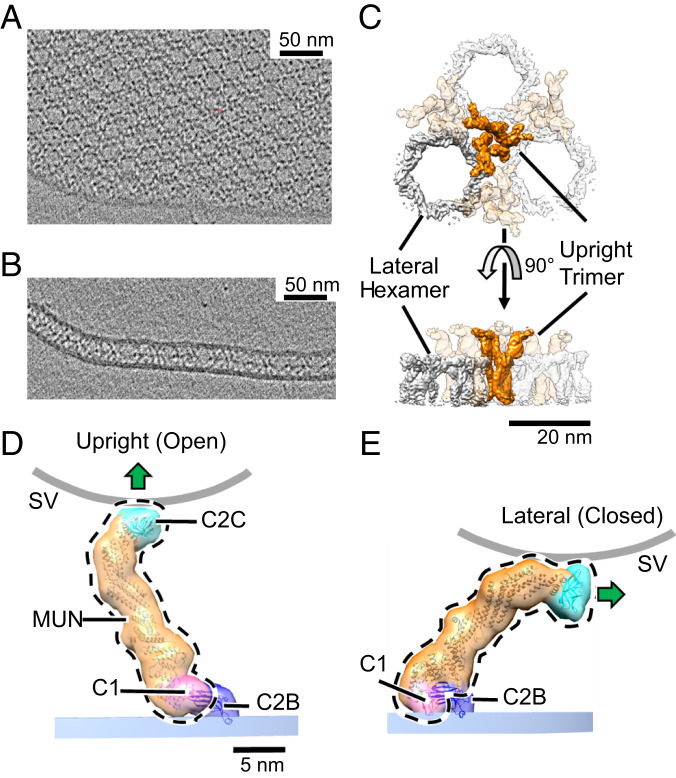
Fig. 1.
Two-dimensional crystal of membrane-bound Munc13C. (A) Representative slice of a reconstructed tomogram showing a view of the crystal perpendicular to the electron beam showing clear hexagonal packing of protein densities (black). (Scale bar 50 nm.) (B) Another slice of the reconstructed tomogram in A shows a region where the crystal plane is oriented parallel to the electron beam. This reveals a layer of protein density sandwiched between two membrane bilayers. (C) Top and side views of the reconstructed crystal 3D map, shown as an isosurface representation. The map shown is a hybrid of two separate 3D reconstructions, focused on upright trimers and lateral hexamers, respectively, to maximize the resolution of each element. The trimers (orange) are formed by Munc13C oligomerized in an open conformation (D) and hexamers (gray color) formed by Munc13C in a closed state (E). (Scale bar: 20 nm.) (D) Upright (open) and (E) lateral (closed) conformations of Munc13C. The two states are related by rigid body rotation of C1 (pink), MUN (orange), and C2C (cyan) domains of the Munc13C molecule (delineated by the black dashed line) relative to the C2B domain (dark blue). This jackknife-like motion rotates C2C and its associated membrane binding surface (shown by a green arrow) almost 90°, disrupting interactions between C2C and the synaptic vesicle membrane (curved gray line). Atomic models in D and E (ribbon diagrams) were obtained by modeling of the AlphaFold predicted Munc13C structure into the ∼10 Å 3D maps (Materials and Methods). For illustration purposes, models are enclosed by semitransparent isosurfaces of corresponding, synthetically generated 3D maps rendered at 20 Å resolution. Experimentally determined 3D maps with superposed atomic models are shown in SI Appendix, Fig. S3. The membrane bilayer region in the reconstruction is indicated by a shaded bar (PM, blue). The putative location of a synaptic vesicle membrane is shown as a thick gray line (SV), whose curvature approximates a sphere with a diameter of 45 nm.