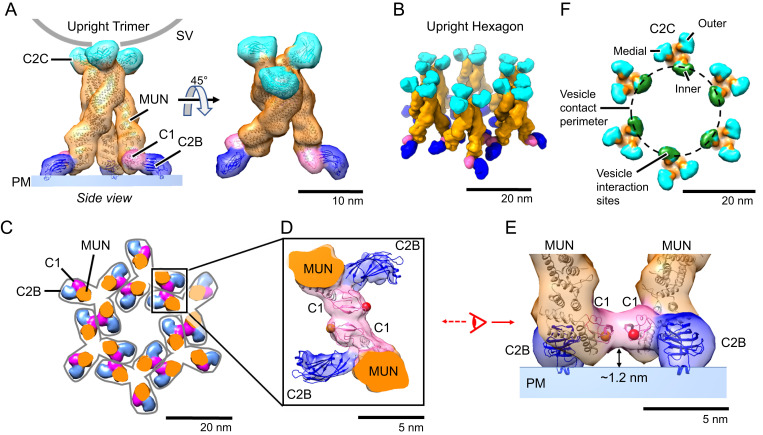
Fig. 2.
Munc13C trimer organization. (A) Upright Munc13C trimer side (Left) and oblique (Right) views (membrane is omitted for clarity). In this figure and the following, except where noted, protein structures are depicted as fitted atomic models (ribbon diagrams) and corresponding synthetic 3D map isosurfaces as in Fig. 1 D and E. For this conformer, the C2C membrane binding surface is oriented upward, allowing it to bind to a vesicle (gray line represents a vesicle with a diameter of 45 nm). The C1 domain is colored pink, C2B in dark blue, MUN domain in orange, and C2C in cyan. (B) Oblique view of the hexagonal array of upright trimers found in the Munc13C crystal (viewing orientation is the same as in A, Right). (C) View of the hexagonal array as in B but showing a horizontal slice focused on the C1/C2B domains, 5 nm above the PM shown in A. Gray outlines delineate the base of each trimer. Domain coloring is the same as in A. Each trimer is connected with neighboring trimers through antiparallel interactions of the C1 domains as shown in D. (D) Expanded view of interactions between neighboring C1 domains (pink). The reconstructed 3D map density indicates a tight contact between the C1 domains of adjacent trimers. Such interactions keep the C1 domains parallel to the membrane as shown in E. This orientation also keeps C1 domains ∼1.2 nm above the membrane with the DAG-binding pocket facing parallel to the membrane (represented with a red sphere, based on the position of the phorbol ester in the structure of PKC C1 bound to the phorbol ester [PDB: 1PTR]). (F) Top view of the trimeric hexamer, focusing on the C2C domains. The dashed line represents the edge of a 45-nm vesicle if it were parked in the middle of the trimeric hexamer, bound to the innermost ring of C2C domains (green). It is likely that medial and outer rings of C2C (cyan) of this assembly are sterically limited in interacting with the parked vesicle.