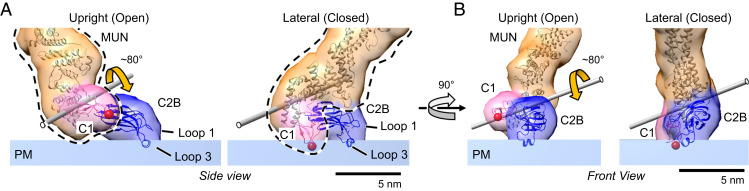
Fig. 4.
Configuration of the N-terminal membrane-binding domains in the open and closed Munc13C conformations. (A) Side view of open (Left) and closed (Right) N-terminal end of Munc13C. The difference between the configurations is based on the jackknife-like action of the C1 (pink), MUN (orange), and C2C (not shown in the figure) domains relative to C2B (blue). The C2B orientation with respect to the bilayer is the same in both conformations, with loop 3 helix inserted into the phospholipid headgroup plane and the beta-strands parallel to the membrane surface. The C1–MUN domains (highlighted by a black dashed line) acts as a rigid unit with a jackknife-like movement about the axis shown by the gray rod and a maximum rotation angle of ∼80° (rotation axis and angle between the two C1–MUN orientations was estimated using the “measure rotation” command in Chimera). During this movement, the orientation of the C1 domain changes dramatically, with the loops that form the DAG-binding pocket (red sphere) making partial contact with the bilayer in the closed conformation. (B) Corresponding front view of the open and closed configurations. Protein structures are depicted as fitted atomic models and corresponding synthetic 3D map isosurfaces as in Figs. 1–3.