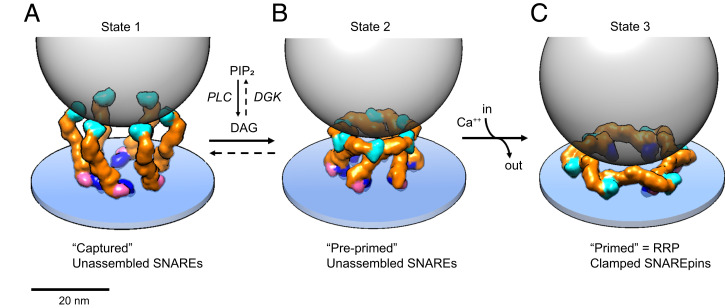
Fig. 6.
Model of how Munc13C may orchestrate vesicle capture, priming, and release. (A) In state 1, the synaptic vesicle (45 nm diameter gray transparent sphere) is captured by the six inner upright Munc13 molecules (other Munc13 molecules that could be involved in this potentially trimeric interaction are not shown for simplicity). In this configuration, each C2C domain is bound to the vesicle membrane and the bottom of the vesicle is now positioned ∼18 nm above the plasma membrane, a separation at which SNAREpins cannot assemble. (B) DAG binding effectively switches the conformation from open (upright) to closed (lateral) rearranging the Munc13C protomers into the closed, hexagon cage (state 2). The hexagon cage now separates the bottom of the synaptic vesicle from the plasma membrane by a minimum of ∼12 nm, still too far for SNAREpins to assemble. SNAREpins are also unable to assemble in state 2 due to autoinhibition. Specifically, the catalytic pocket on the surface of the MUN domain needed to open the closed Syntaxin1A–Munc18 complex is occluded by the C2C domain of the neighboring protomer. (C) State 3 is the RRP, consisting of primed vesicles with approximate half-zippered SNAREpins clamped by Synaptotagmins. State 2 is proposed to transition to state 3 as the result of an influx of Ca2+ binding to Munc13 C2B domains and causing them to rotate toward the plasma membrane when they insert Ca2+-binding loop 1 into the bilayer along with previously inserted loop 3. This is also expected to rotate MUN–C2C toward and directly against the plasma membrane. The orientation of Munc13 on the plasma membrane is unknown but is based on an illustrative model (SI Appendix, Fig. S9). The actual orientation might be more radially open. The bottom of the synaptic vesicle is now positioned ∼4 to 5 nm above the PM surface, with SNAREpins partially assembled and forming complexes with Synaptotagmin-1 C2B domains, thus priming the vesicle for ready release when the synapse is next activated.