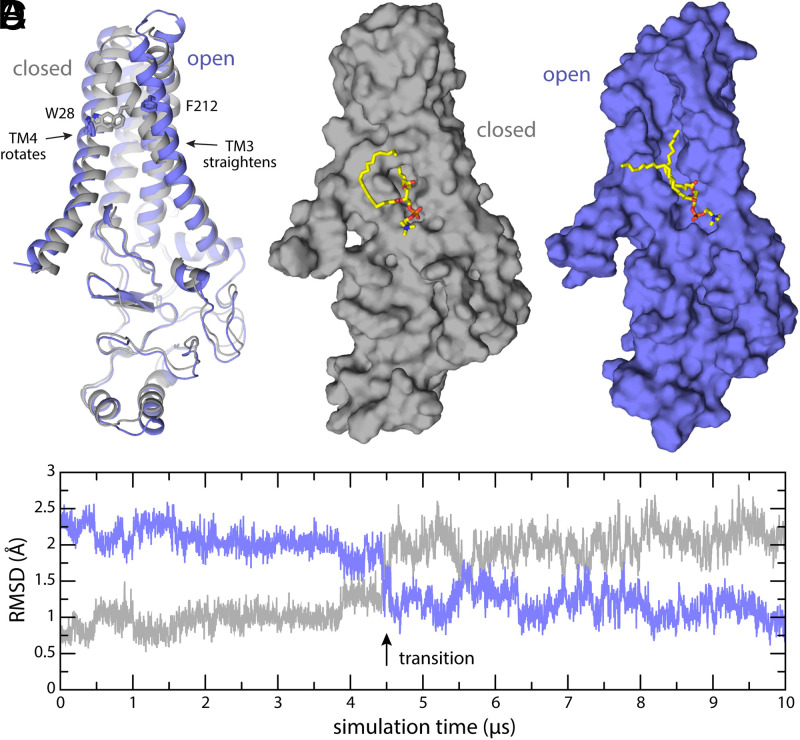
Fig. 6.
Putative mechanism of gating of the PCoA-binding pocket. (A) The experimental structure of apo-state DHHC20 (9) (gray) is compared with the final snapshot of a 10-µs MD trajectory (purple). The trajectory reveals the transformation of the binding pocket into an open cleft through local but consequential changes in helices TM3 and TM4. For example, the side chains of Trp28 and Phe212, which are in direct contact and contribute to occlude the binding pocket in the closed state, become separated by 16 Å in the putatively open state. (B and C) Same as A on a surface representation. Membrane and solvent are omitted for clarity, except the POPC lipid molecule that partially occupies the binding pocket in absence of PCoA. (D) Time-series of the RMSD between the snapshots observed in simulation and either the experimental structure of apo-state DHHC20 (gray) or the putatively open state depicted in A and C. For clarity, the RMSD is quantified only for the backbone of the transmembrane span. The approximate time point in which the opening transition occurs is indicated.