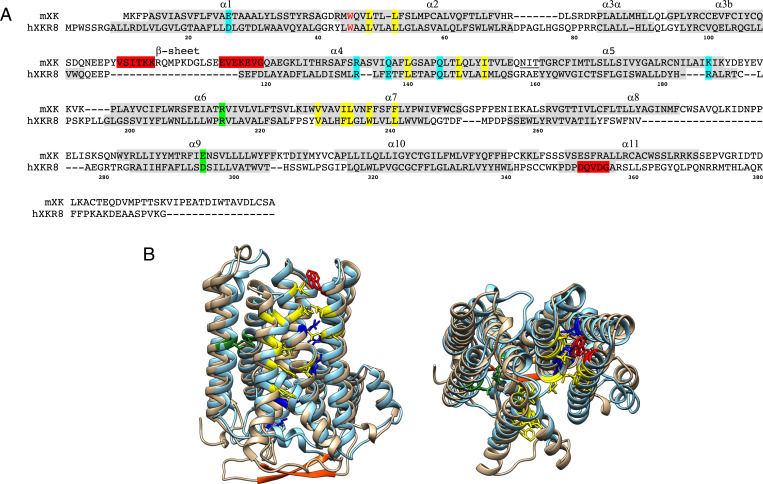
Fig. 7.
Conserved structure between Xkr8 and Xk. (A) The amino acid sequences of mouse Xk and human XKR8 are aligned with α-helices numbered. Helices α1 to α10 are in the membrane. The residues (R214 and D295) highlighted in green form a salt bridge in XKR8 and are mutated in XK of McLeod syndrome patients. The conserved charged residues in the transmembrane are highlighted in blue, while the hydrophobic residues forming the cleft for a phospholipid in XKR8 are highlighted in yellow. The tryptophan residue (W45) that serves as a gate keeper (10) is shown in red. A caspase-recognition site in XKR8 and the β-hairpin in Xk are highlighted in red. Numbers at the bottom indicate the position of amino acid of XKR8. A putative N-glycosylation site at the extracellular loop between α4 and α5 of Xk is underlined. (B) Side (left) and top (right) views of the structure of mouse Xk (tan), predicted by AlphaFold, was superimposed on human XKR8 structure (cyan) (10). The residues were colored as above. The C-terminal tail regions of XKR8 and Xk were not included since the cryo-electron microscopy structure of hXKR8 did not reveal it (10).