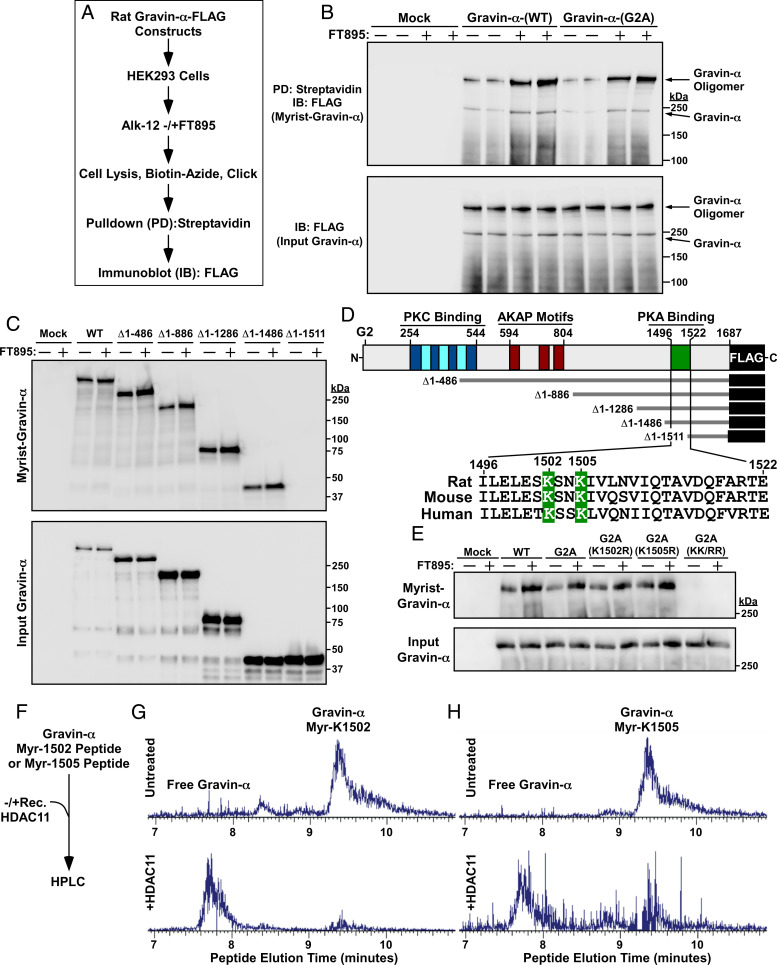
Fig. 3.
HDAC11 demyristoylates two lysines in the PKA binding domain of gravin-α. (A) Schematic depiction of the experiment to assess myristoylation of ectopically expressed gravin-α constructs. (B) Immunoblot analysis of homogenates of HEK293 cells transfected with the indicated FLAG-tagged gravin-α constructs and treated with vehicle control (−) or 10 μM FT895 for 12 h. Myrist, myristoylated. (C) Immunoblot analysis of homogenates of HEK293 cells transfected with the indicated constructs for N-terminally truncated, FLAG-tagged gravin-α and treated with vehicle control (−) or 10 μM FT895 for 12 h. (D) Schematic representation of gravin-α, gravin-α constructs, and the conserved lysine myristoylation sites embedded in the PKA binding domain of gravin-α. (E) Immunoblot analysis of homogenates of HEK293 cells transfected with constructs for gravin-α harboring the indicated amino acid substitutions and treated with vehicle control (−) or 10 μM FT895 for 12 h. (F) Schematic depiction of the experiment to address whether HDAC11 is capable of directly demyristoylating lysine-1502 and lysine-1505 of gravin-α. (G) LC-MS traces of myristoyl-lysine-1502 peptide and free peptide of gravin-α before and after treatment with recombinant HDAC11 for 1.5 h at 37 °C. (H) LC-MS traces of myristoyl-lysine-1505 peptide and free peptide of gravin-α before and after treatment with recombinant HDAC11 for 1.5 h at 37 °C. For G and H, peaks were searched using Xcaliber software. Myr, myristoyl. Peaks were searched using Xcaliber software. Please visit Figshare for a higher-resolution version (10.6084/m9.figshare.19082813).