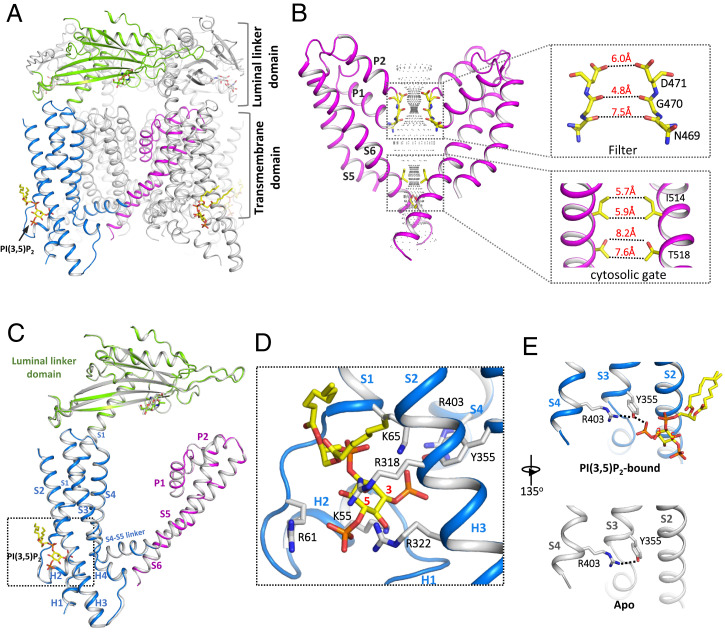
Fig. 2.
Structure of closed TRPML1 in apo and PI(3,5)P2-bound states. (A) Side view of a cartoon representation of the PI(3,5)P2-bound closed TRPML1 structure with the three major domains from the front subunit colored (blue for S1 to S4, green for the luminal linker domain, and magenta for the pore domain). (B) The closed ion-conduction pore of TRPML1 with only two diagonal subunits shown for clarity. The central ion pathway is marked with dotted mesh. Key gating and filter residues are shown as sticks. (Insets) Zoomed-in views of the filter and the cytosolic gate. (C) Structural comparison of a single subunit between apo (gray) and PI(3,5)P2-bound (colored) TRPML1. (D) Zoomed-in view of the PI(3,5)P2-binding site with key ligand-interacting residues shown. The red numbers mark the C3 and C5 positions of inositol. (E) H-bonding interaction between R403 and Y355 in the presence and absence of PI(3,5)P2.